FIGURE 8:
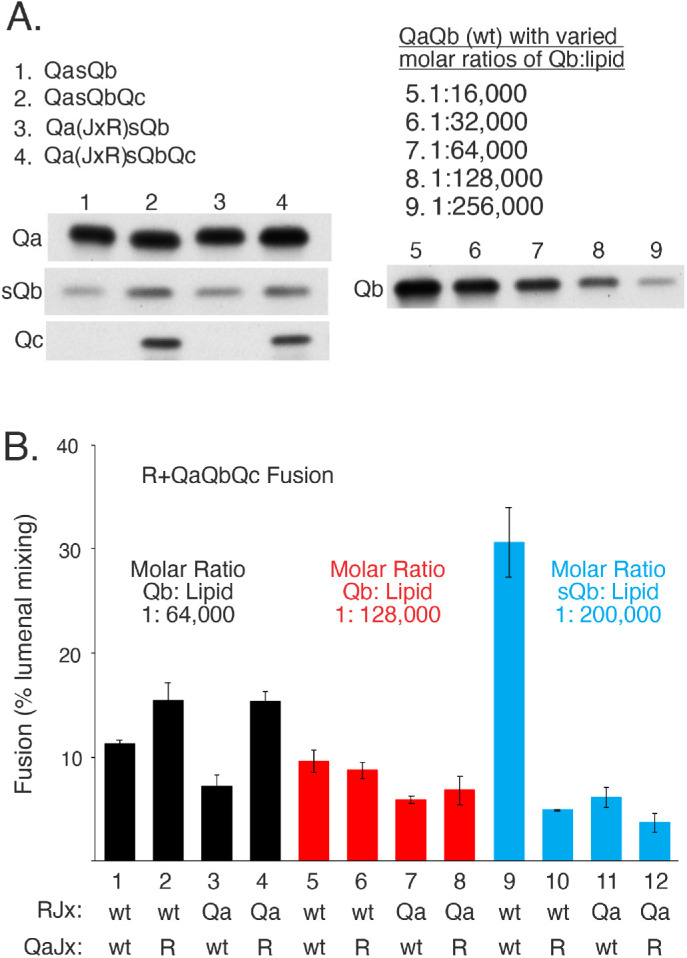
R and QaQbQc proteoliposomes fuse with little sensitivity to Jx swap, but R and QasQbQc proteoliposome fusion is strongly inhibited by Jx-domain swap between R and Qa. (A) Proteoliposome preparation. R and R(JxQa) proteoliposomes and QasQbQc and Qa(JxR)sQbQc proteoliposomes were prepared with Ypt7 as described in Materials and Methods, but with 5× the molar concentrations of sQb and Qc (where present) as Qa in the initial mixed micellar solution to drive SNARE complex assembly. Unincorporated SNAREs are removed during proteoliposome flotation. QaQb proteoliposomes were also prepared as described, with a 1:16,000 molar ratio of Qa:lipid, but with the indicated molar ratios of wild-type Qb to lipid, serving as standards for the level of sQb assembly into proteoliposomes. After isolation and adjusting each to 2 mM lipid, equivalent volumes of the indicated proteoliposomes were analyzed by SDS gel and immunoblot for their content of Qa, Qc, and sQb or Qb. sQb and Qb samples were analyzed on the same gel and blot. (B) Fusion of R and 3Q proteoliposomes. R and Qa were at 1:16,000 molar ratios to lipid and were either wild-type or R(JxQa) or Qa(JxR). Wild-type Qb was incorporated at 1:64,000 and 1:128,000 molar ratios to lipid to approach the lower levels of sQb incorporated. Fusion was assayed as described in Figure 7, but without any soluble SNARE additions, Sec17, or Sec18. Mean and SDs of fusion for triplicate reactions after 20 min are shown. See Supplemental Figure S4 for typical kinetic data.
