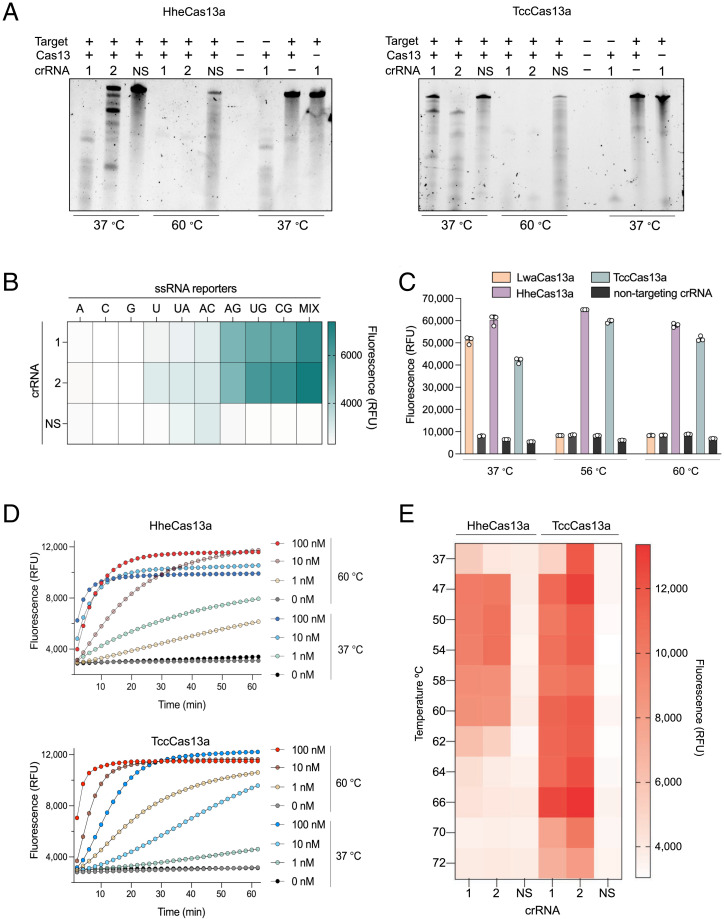
Fig. 2.
Characterization of cis and trans cleavage activities of thermophilic HheCas13a and TccCas13a. (A) Representative denaturing gels showing the targeted in vitro RNase cleavage activity of HheCas13a and TccCas13a proteins when incubated with ssRNA targets and different crRNAs at different temperatures. NS, nonspecific crRNA control. (B) TccCas13a collateral cleavage preference for the ssRNA reporter. Reactions consisting of TccCas13a and its respective cognate crRNAs or NS were performed in the presence of ssRNA targets and one of 10 ssRNA reporters. Data are shown as mean (n = 3). Reactions were incubated at 56 °C, and the end-point fluorescent signal was measured after 30 min. ssRNA reporter sequences are shown on top of the heat map. A, poly A reporter; C, poly C reporter; G, poly G reporter; U, poly U reporter; UA, LwaCas13a reporter; AC, 3(AC) reporter; AG, 3(AG) reporter; UG, 3(UG) reporter; CG, 3(CG) reporter; Mix, mix reporter (UGACGU) (SI Appendix, Table S6). (C) End-point activity of LwaCas13a, HheCas13a, and TccCas13a at different temperatures using their preferred reporter (SI Appendix, Table S6 for LwaCas13a reporter). One crRNA and a nonspecific crRNA were tested for each. Values are shown as mean ± SD and represent end-point fluorescence at 30 min. (D) Measurement of real-time fluorescence output comparing the detection activity of HheCas13a and TccCas13a at three different target RNA concentrations (100, 10, and 1 nM) and two temperatures (37 and 60 °C). Data are shown as mean (n = 3). (E) End-point activity of HheCas13a and TccCas13a at different temperatures using their preferred reporter after 30 min. Two crRNAs and a nonspecific crRNA were tested for each. Data are shown as mean (n = 3). RFU, relative fluorescence unit.