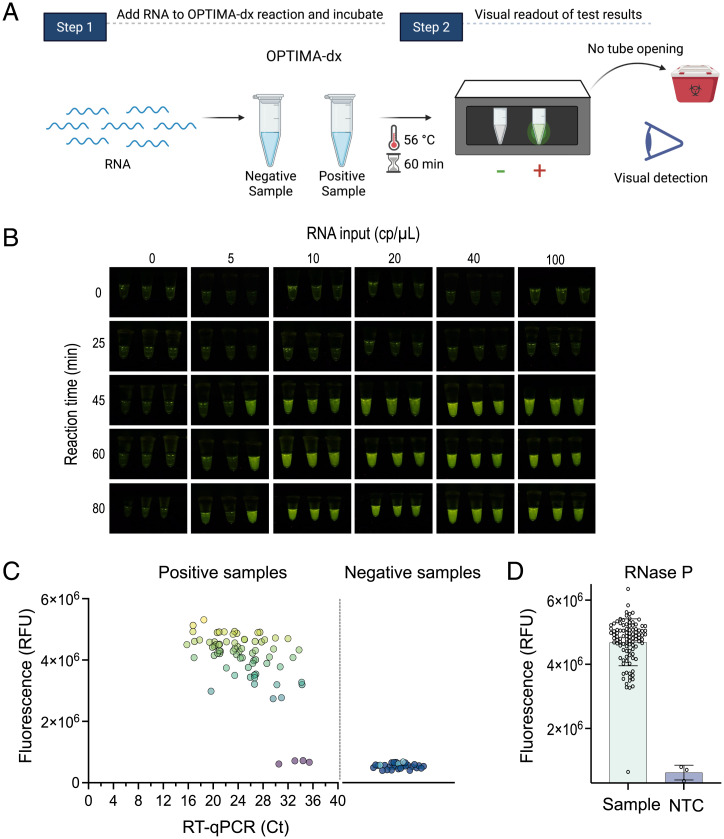
Fig. 5.
Evaluation of OPTIMA-dx for the detection of SARS-CoV-2. (A) Schematic representation of SARS-CoV-2 RNA detection in one-pot assays and visual detection using the P51 Molecular Fluorescence Viewer. As the test is performed in a single pot, there is no need to open the reaction tube, so it can be discarded without opening, thus avoiding the possibility of contamination at the POC site. (B) Assessment of the sensitivity of OPTIMA-dx and the effect of reaction incubation time on performance using fluorescence-based visual detection. Fluorescence rises above background after 45 min, with little improvement as time increases. Three replicates were performed for each treatment. (C) SARS-CoV-2 detection from 100 clinical COVID-19 samples with one-pot RT-LAMP TccCas13a detection assay. qRT-PCR Ct plotted against fluorescence readout from the detection of SARS-CoV-2–positive samples (n = 73) and SARS-CoV-2–negative samples (n = 27). Detection reactions were incubated at 56 °C, and the end-point fluorescence signal was measured after 1 h. Light-blue data points in negative samples represent no template controls (NTC). (D) Detection of RNase P internal control with one-pot RT-LAMP TccCas13a detection assay. All 100 clinical samples in A were tested for the detection of the RNase P gene. Detection reactions were incubated at 56 °C, and the end-point fluorescence signal was measured after 1 h. RFU, relative fluorescence unit.