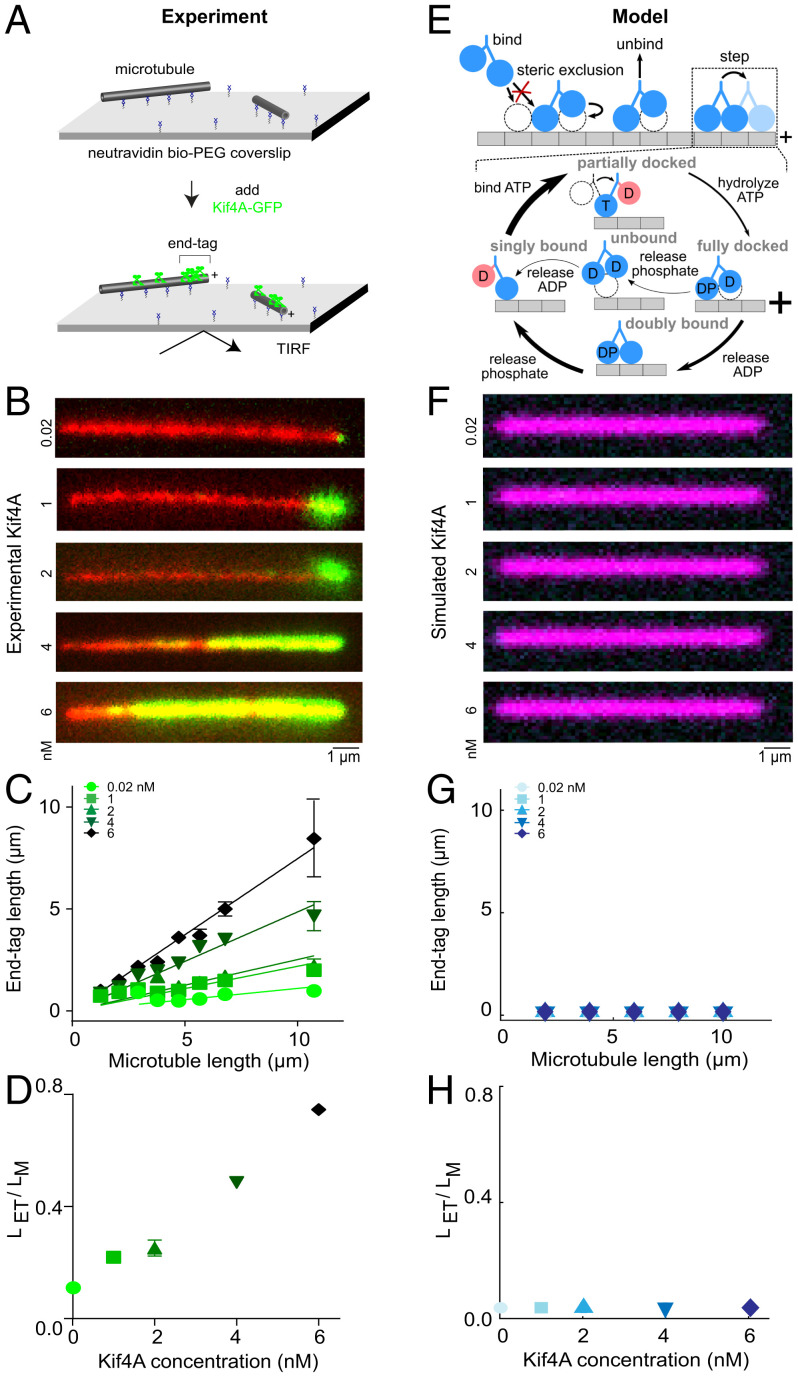
Fig. 1.
The kinesin-4 motor Kif4A forms microtubule-length–dependent end tags, but a minimal motor model does not reproduce the experimental observations. (A) Schematic of the in vitro assay used to study Kif4A-GFP (green) on single microtubules (gray). (B) Representative fluorescence micrographs showing end-tag formation with Kif4A-GFP concentration from 0.02 to 6 nM. Images show X-rhodamine–labeled microtubules (red) with Kif4A-GFP (green). (C) End-tag length versus microtubule length in assays with Kif4A-GFP concentration from 0.02 to 6 nM: 0.02 nM (slope 0.11 ± 0.02), 1 nM (slope 0.22 ± 0.02), 2 nM (slope 0.25 ± 0.03), 4 nM (slope 0.49 ± 0.02), and 6 nM (slope 0.75 ± 0.02). (D) Slope (end-tag length divided by microtubule length) versus Kif4A concentration. (E) Model overview. Motors can bind, unbind, and step, constrained by steric interactions. Inset, model mechanochemical cycle. See SI Appendix and previous work (32). (F) Simulated fluorescence images created from the model using 10-µm-long microtubules and with Kif4A concentration from 0.02 to 6 nM. (G) Simulated end-tag length versus microtubule length. (H) Slope (simulated end-tag length divided by microtubule length) versus Kif4A concentration.