Abstract
Context
The genetic architecture of isolated hypogonadotropic hypogonadism (IHH) has not been completely defined.
Objective
To determine the role of copy number variants (CNVs) in IHH pathogenicity and define their phenotypic spectrum.
Methods
Exome sequencing (ES) data in IHH probands (n = 1394) (Kallmann syndrome [IHH with anosmia; KS], n = 706; normosmic IHH [nIHH], n = 688) and family members (n = 1092) at the Reproductive Endocrine Unit and the Center for Genomic Medicine of Massachusetts General Hospital were analyzed for CNVs and single nucleotide variants (SNVs)/indels in 62 known IHH genes. IHH subjects without SNVs/indels in known genes were considered “unsolved.” Phenotypes associated with CNVs were evaluated through review of patient medical records. A total of 29 CNVs in 13 genes were detected (overall IHH cohort prevalence: ~2%). Almost all (28/29) CNVs occurred in unsolved IHH cases. While some genes (eg, ANOS1 and FGFR1) frequently harbor both CNVs and SNVs/indels, the mutational spectrum of others (eg, CHD7) was restricted to SNVs/indels. Syndromic phenotypes were seen in 83% and 63% of IHH subjects with multigenic and single gene CNVs, respectively.
Conclusion
CNVs in known genes contribute to ~2% of IHH pathogenesis. Predictably, multigenic contiguous CNVs resulted in syndromic phenotypes. Syndromic phenotypes resulting from single gene CNVs validate pleiotropy of some IHH genes. Genome sequencing approaches are now needed to identify novel genes and/or other elusive variants (eg, noncoding/complex structural variants) that may explain the remaining missing etiology of IHH.
Keywords: isolated hypogonadotropic hypogonadism, genetics, copy number variants, missing heritability, phenotypes
Missing Heritability in Isolated Hypogonadotropic Hypogonadism
Despite substantial progress in genomic technologies, current molecular testing in rare Mendelian disorders typically identifies a putative causative variant in only ~50% of cases (1, 2). Isolated hypogonadotropic hypogonadism (IHH) is a rare Mendelian disorder that is caused by gonadotropic releasing hormone (GnRH) deficiency and results in hypogonadism and infertility. Patients with IHH present with incomplete sexual maturation with or without anosmia (1). Even though > 60 genes have been implicated in IHH, ~50% of the cases remain genetically undiagnosed (1). A portion of this missing genetic heritability likely lies in novel genes that require larger sample size for discovery or in mutations currently not easily tractable in exome sequencing (ES), such as copy number variants (CNVs) and variants in the noncoding region of the genome.
Copy Number Variants in Isolated Hypogonadotropic Hypogonadism
CNVs are a class of structural variants that result in either loss (deletions) or gain (duplications) of genetic material (> 50 base-pairs of genomic DNA). CNVs have traditionally been captured with karyotypes or chromosomal microarrays and investigation of IHH with these technologies previously has led to important genetic discoveries (3-6). While large ANOS1 deletions lead to IHH (7), and prior CNV studies in a subset of IHH genes show an overall prevalence of ~1%, the contribution of CNVs in all IHH-associated genes has not been systematically studied (8-10). Although prior studies used contemporary techniques available at that time, the CNV capture methods employed are of low resolution (karyotypes capture CNVs > 5 to 10 MB in size; and chromosomal microarrays are typically limited to CNV > 100 kb (11)). In addition, chromosomal microarrays run the risk of missing genomic regions that are not densely covered by the designed SNPs (12). Hence, the low prevalence of CNVs in prior IHH studies can be to the evaluation of a subset of causative IHH genes and the low-resolution tools that have been used to call CNVs (8, 9).
Advances in tools analyzing next-generation sequencing data now allow precise characterization of CNVs. These advanced analytic pipelines can detect CNVs of a smaller size compared to microarrays. Recently, our team has benchmarked GATK-gCNV/ES analysis against genomes and microarray in the same samples (13). These analyses have demonstrated > 95% sensitivity for rare CNVs that span > 4 exons, > 90% positive predictive value, and captured all CNVs detected by microarray (13). In addition, the application of the GATK-gCNV pipeline to ES data in other rare Mendelian disorders has recently demonstrated pathogenic CNVs (11). In this study, the gCNV-GATK pipeline was employed to call CNVs from ES data in a large cohort of 1394 IHH patients to precisely define the contribution of CNVs in known genes to the IHH genetic architecture.
Materials and Methods
Study Participants
All research protocols were approved by the institutional review board at the Massachusetts General Hospital/Partners Healthcare. Participants provided written informed consent for participation.
Phenotypic Evaluation
A total of 1394 IHH (n = 706 with Kallmann syndrome [KS; IHH with anosmia] and n = 688 normosmic IHH [nIHH]) patients (990 males and 404 females) were recruited at the Reproductive Endocrine Unit/ Massachusetts General Hospital (MGH). IHH was defined by: (i) absent/incomplete puberty by age 18 years of age; (ii) serum testosterone < 100 ng/dL (men) or estradiol < 20 pg/mL (women) with low/normal serum gonadotropins; (iii) otherwise normal anterior pituitary function; (iv) normal serum ferritin concentrations; and (v) normal magnetic resonance imaging of the pituitary region (14). Both self-reported olfaction as well as University of Pennsylvania Smell Identification Test (UPSIT) scores were used to classify KS and nIHH (15). Clinical charts and patient questionnaires (300 questions), with regard to reproductive and nonreproductive history, were reviewed. Nonreproductive features involved bone, face/head, cardiovascular, hearing, neurodevelopmental, neurologic, skin, and eye disorders. Family history was obtained and, whenever possible, family members were recruited by our study team.
Genomic DNA Extraction
Peripheral blood samples were collected from all participants to extract genomic DNA.
Exome Sequencing
Exome sequencing (ES) was performed on IHH participants (N = 1394) and their family members (N = 1092) at the Broad Institute (Cambridge, Massachusetts, USA; n = 2386) or the Yale Center for Mendelian Genomics (Orange, Connecticut, USA; n = 100). Alignment of the ES data against the reference genome (hg19), initial quality control, and variant calling algorithms were applied using GATK best practices (Broad Institute), as previously described (16).
Copy Number Variant Calling
CNV calls were conducted using the GATK-gCNV pipeline, a high sensitivity Bayesian model for CNV calling from ES data. GATK-gCNV adjusts for known bias factors of exome capture and sequencing, such as GC content and mappability, while also controlling for other technical and systematic differences. Raw sequencing files were compressed into read counts over the set of exons comprising canonical protein-coding transcripts and used as input. A principal component analysis–based approach was implemented on observed read counts to highlight samples on different capture kits, followed by hierarchical clustering to curate batches of samples for parallel processing (Supplemental References (17)). Comprehensive filtering metrics derived from the underlying Bayesian model were included for each detected variant, which are then tuned to balance between sensitivity and specificity. Rare CNVs (population frequency < 1%) were prioritized. Monogenic full gene and intragenic exonic CNVs were defined by breakpoints upstream/downstream and within of the open reading frame of the gene, respectively. Monogenic partial CNVs were defined by one breakpoint partially interrupting the coding region of the gene and multigenic CNVs spanned multiple genes including the gene of interest.
Copy Number Variant Confirmation
To confirm the candidate CNVs, TaqMan assays (Supplemental Table 1 (17)) were used for genotyping each locus. TaqMan Copy Number Reference Assay (genotyping human the RNase P gene) was used as reference. Experiments were performed according to the manufacturer’s instructions and StepOnePlus instrument (Applied Biosystems Inc., USA) was used for quantitative polymerase chain reaction (qPCR). For the CNV analysis, CT values were obtained from StepOne Software v.2.2.2 (Applied Biosystems Inc., USA) and then imported to Copy Caller Software v.2.0 (Applied Biosystems Inc., USA) to determine copy numbers.
Rare Variant Association Testing
The total number of alternate and reference alleles across all IHH genes were aggregated into a single alternate allele count (AAC) and reference allele count (RAC) per group. The AACs and RACs were then used in a single rare variant burden test between the IHH participants and gnomAD controls (Fisher exact test). Statistical significance was defined by a P value with a cutoff < 0.002 (multiple testing correction for 13 genes/26 alleles).
Juxtaposing Copy Number Variant With Single Nucleotide Variant/Indel Data Analysis
Single nucleotide variant (SNV)/indel calling and joint genotyping were performed using GATK components HaplotypeCaller and GenotypeGVCFs. VCF files were annotated using SnpSift 4.3k and Ensembl VEP release 93. Sanger sequencing was used to confirm the SNVs/indels. IHH cases with the following types of SNV/indels in the 62 IHH genes were determined to be “solved” (Supplemental Table 2 (17)): (i) rare homozygous or compound heterozygous SNVs/indels (overall minor allele frequency [MAF] < 1% in gnomAD controls (18)) in reported autosomal recessive genes; (ii) rare hemizygous SNVs/indels (MAF < 0.1%) in reported X-linked recessive genes; and (iii) rare heterozygous SNVs/indels (MAF < 0.1%) in reported autosomal dominant/indeterminant inheritance genes. Since several IHH genes in this last category have been reported in reports without robust burden testing, rare variant association testing (RVAT) was performed for each gene (including domain-based testing whenever applicable). Variants that showed enrichment in the IHH cohort were considered causative.
Results
Prevalence of CNVs in Isolated Hypogonadotropic Hypogonadism
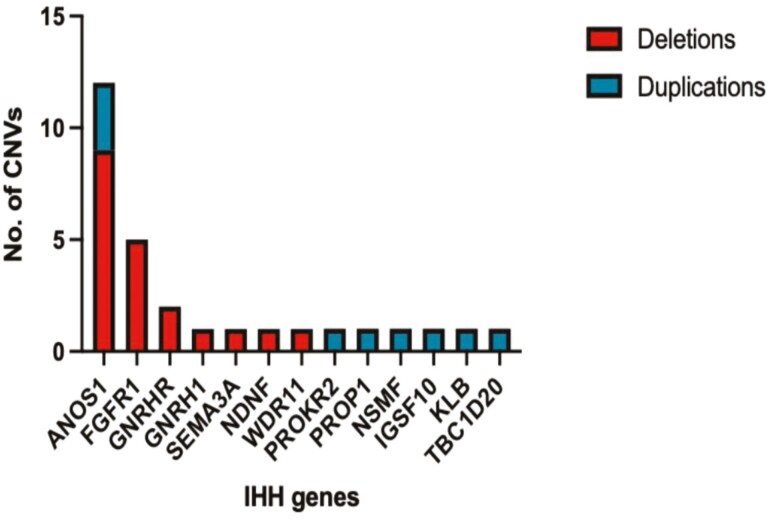
ES of all IHH probands (N = 1394) was analyzed using the GATK-gCNV pipeline for rare CNVs in 62 genes implicated in IHH (Supplemental Table 2 (17)). A total of 37/1394 IHH probands harbored CNVs in 15/62 IHH genes. Confirmation with RT-qPCR was performed for 33/37 CNVs and was not feasible in 4 CNVs due to the increased number of masked bases in their genomic regions that prevented the design of optimal primers. The confirmation rate was 88% (29/33) highlighting the high accuracy of the gCNV pipeline. Thus, an overall prevalence of 2% (29/1394) for confirmed rare CNVs affecting 13 known IHH genes was established in a large cohort of IHH subjects (Fig. 1). In keeping with the higher number of males in the entire IHH cohort, the CNV prevalence was also relatively higher in males (25 CNVs in 990 males; ~2.5%) compared with females (4 CNVs in 404 females (~1%). Since ANOS1-CNVs (X-linked gene) accounted for a significant number of CNVs in males, the CNV prevalence was examined for autosomal genes. Although this analysis showed higher autosomal CNVs in males (1.4%) compared with females (0.7%), the difference was not statistically different (P value 0.46).
Figure 1.
Rare deletions and duplications detected in IHH-associated genes: ANOS1, FGFR1, GNRHR, GNRH1, SEMA3A, NDNF, WDR11, PROKR2, PROP1, NSMF, IGSF10, KLB, TBC1D20.
CNV Characteristics
Confirmed CNVs ranged from 4.9 KB to 9.6 MB in size (median size of 207 KB). Most CNVs were deletions (n = 20), of which the majority (13/20) were multigenic (Table 1). Five multigenic deletions spanned the ANOS1 gene, 5 spanned the FGFR1 gene, and each of the remaining 3 multigenic deletions spanned only 1 IHH gene (GNRHR, NDNF, and WDR11, respectively). The remaining 7/20 deletions were monogenic, disrupting only a single IHH gene: 1 full gene deletion spanned the SEMA3A locus; 2 deletions were intragenic exonic (affecting 7 ANOS1 exons); and 4 deletions partially affected the genes of ANOS1(N = 2), GNRHR (N = 1), and GNRH1 (N = 1). Overall, of the 20 deletions discovered, the majority disrupted either ANOS1 (N = 9) or FGFR1 (N = 5). Nine duplications were detected in 9 IHH probands (Table 1), of which 5 were multigenic and encompassed the PROP1, NSMF, KLB, TBC1D20, and IGSF10 genes. Four duplications disrupted only a single IHH gene: 3 intragenic exonic duplications of ANOS1 and 1 full gene duplication of PROKR2. All identified deletions and intragenic duplications were deemed pathogenic by the American College of Medical Genetics and Genomics (ACMG) and the Clinical Genome Resource (ClinGen) recommendations (19) (Supplemental Table 3 (17)), while additional studies are required to define the pathogenicity of the reported full gene duplications.
Table 1.
Copy number variants spanning known IHH genes
| Gene | Chr | Start | End | Size | Zygosity | CNV type | Monogenic full gene | Monogenic Partial | Intragenic (N of exons) | Multi-genic | Inheritance | Dx | Gender |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ANOS1 | chrX | 2771923 | 9734102 | 6.9 MB | Hem | Deletion | No | No | No | Yes | De novo | KS | Male |
| ANOS1 | chrX | 2771923 | 8536514 | 5.7 MB | Hem | Deletion | No | No | No | Yes* | Unknown | KS | Male |
| ANOS1 | chrX | 6966863 | 8591808 | 1.6 MB | Hem | Deletion | No | No | No | Yes* | Unknown | KS | Male |
| ANOS1 | chrX | 7137374 | 8700325 | 1.5 MB | Hem | Deletion | No | No | No | Yes | X-linked | KS | Male |
| ANOS1 | chrX | 6966863 | 8700325 | 1.7 MB | Hem | Deletion | No | No | No | Yes | X-linked | KS | Male |
| ANOS1 | chrX | 8521895 | 8591808 | 69 KB | Hem | Deletion | No | No | Yes (7) | No | De novo | KS | Male |
| ANOS1 | chrX | 8521895 | 8591808 | 69 KB | Hem | Deletion | No | No | Yes (7) | No | Unknown | KS | Male |
| ANOS1 | chrX | 8521895 | 8700325 | 178 KB | Hem | Deletion | No | Yes | No | No | X-linked | KS | Male |
| ANOS1 | chrX | 8538442 | 8700325 | 161 KB | Hem | Deletion | No | Yes | No | No | X-linked | KS | Male |
| FGFR1 | chr8 | 29920430 | 39537842 | 9.6 MB | Het | Deletion | No | No | No | Yes | De novo | KS | Male |
| FGFR1 | chr8 | 37553171 | 42050826 | 4.4 MB | Het | Deletion | No | No | No | Yes | Unknown | KS | Male |
| FGFR1 | chr8 | 35401812 | 40555017 | 5.1 MB | Het | Deletion | No | No | No | Yes | Unknown | KS | Male |
| FGFR1 | chr8 | 36641744 | 39114959 | 2.4 MB | Het | Deletion | No | No | No | Yes | Unknown | nIHH | Male |
| FGFR1 | chr8 | 37553171 | 39142533 | 1.5 MB | Het | Deletion | No | No | No | Yes | Unknown | KS | Male |
| GNRHR | chr4 | 65155330 | 70391829 | 5.2 MB | het | Deletion | No | No | No | Yes | In trans with SNV | nIHH | Male |
| GNRHR | chr4 | 68610188 | 68621901 | 11 KB | Hom | Deletion | No | Yes | No | No | AR | nIHH | Male |
| GNRH1 | chr8 | 25278991 | 25282653 | 3.6 KB | Hom | Deletion | No | Yes | No | No | AR | nIHH | Male |
| SEMA3A | chr7 | 83587561 | 83824314 | 236 KB | Het | Deletion | Yes | No | No | No | Unknown | KS | Male |
| NDNF | chr4 | 119944487 | 126408858 | 6.4 MB | het | Deletion | No | No | No | Yes | Unknown | nIHH | Male |
| WDR11 | Chr10 | 115312639 | 124097792 | 8.7 MB | Het | Deletion | No | No | No | Yes | De novo | KS | Female |
| ANOS1 | chrX | 8521895 | 8591808 | 69913 | Het | Duplication | No | No | Yes (7) | No | Unknown | KS | Female |
| ANOS1 | chrX | 8521895 | 8591808 | 69 KB | Het | Duplication | No | No | Yes (7) | No | Unknown | nIHH | Mlale |
| ANOS1 | chrX | 8553210 | 8591808 | 38 KB | Het | Duplication | No | No | Yes (3) | No | Unknown | KS | Male |
| PROKR2 | chr20 | 5281748 | 5295120 | 13 KB | Het | Duplication | Yes (CN = 5)*** | No | No | No | Unknown | KS | Male |
| PROP1** | chr5 | 177419138 | 177573377 | 154 KB | Het | Duplication | No | No | No | Yes | Unknown | niHH | Female |
| NSMF | chr9 | 140218079 | 140396276 | 178 KB | Het | Duplication | No | No | No | Yes | De novo | KS | Male |
| IGSF10 | chr3 | 150804487 | 151176594 | 372 KB | Het | Duplication | No | No | No | Yes (CN = 4)*** | Unknown | nIHH | Male |
| KLB | chr4 | 39435732 | 39512578 | 76 KB | Het | Duplication | No | No | No | Yes* | Unknown | nIHH | Male |
| TBC1D20** | chr20 | 388596 | 480675 | 92 KB | Het | Duplication | No | No | No | Yes | AD | KS | female |
The table shows the IHH gene affected by CNVs; the chromosomal breakpoints that defined the detected CNV; the size of the CNVs; their zygosity; type of CNVs (deletions/duplications; monogenic full gene, monogenic partial, intragenic exonic, and multigenic).
Abbreviations: AD, autosomal dominant; AR, autosomal recessive; CNV, copy number variant; IHH, isolated hypogonadotropic hypogonadism; KS, Kallmann syndrome (ie, IHH with anosmia); nIHH, normosmic IHH.
*Indicates the genes that were partially disrupted by a breakpoint of multigenic CNVs; their inheritance pattern, the diagnosis (Dx) of the probands, and the gender of the probands affected by the CNVs.
** Genes associated with syndromic forms of IHH (PROP1 is associated with combined pituitary hormone deficiency and TBC1D20 with Warburg Micro syndrome).
*** The duplication of IGSF10 led to 4 copies of the gene (copy number/CN = 4) and the duplication of PROKR2 to 5 copies (CN = 5).
CNV Burden in IHH vs Control Datasets
Τhe prevalence of CNVs in the 13 genes affected by CNVs in this cohort was examined in the gnomAD population dataset (N = 10 847) (18). Rare CNVs were reported in 7/13 genes within the gnomAD cohort while 6/13 genes lacked CNVs in this control population (Supplemental Table 4 (17)). The IHH cohort was enriched for both rare deletions and duplications compared to controls (Table 2). These observations support the pathogenic likelihood of the detected CNVs in the IHH cohort.
Table 2.
Rare copy number variant (deletions and duplications) association testing in IHH subjects compared with gnomAD controls
| Type of rare CNVs | IHH cohort (N = 1394) | gnomAD (N = 10 847) | |||||
|---|---|---|---|---|---|---|---|
| Affected alleles | Total alleles | Prevalence | Affected alleles | Total alleles | Prevalence | P value | |
| Deletions | 20 | 2784 | 0.007 | 54 | 21 694 | 0.002 | 0.00002* |
| Duplications | 9 | 2784 | 0.003 | 6 | 21 694 | 0.0002 | 0.00002* |
| Total CNVs | 29 | 2784 | 0.01 | 60 | 21 694 | 0.002 | 0.00001* |
Number of affected alleles with rare deletions and duplications in the IHH cohort compared to gnomAD controls. The IHH cohort is significantly enriched for all types of rare CNVs (both deletions and duplications) in IHH genes compared with controls.
Abbreviations: CNV, copy number variant; IHH, isolated hypogonadotropic hypogonadism.
* Statistical significance was defined by a P value with a cutoff < 0.002 (multiple testing correction for 13 genes/26 alleles).
Inheritance of Identified CNVs in IHH Cases
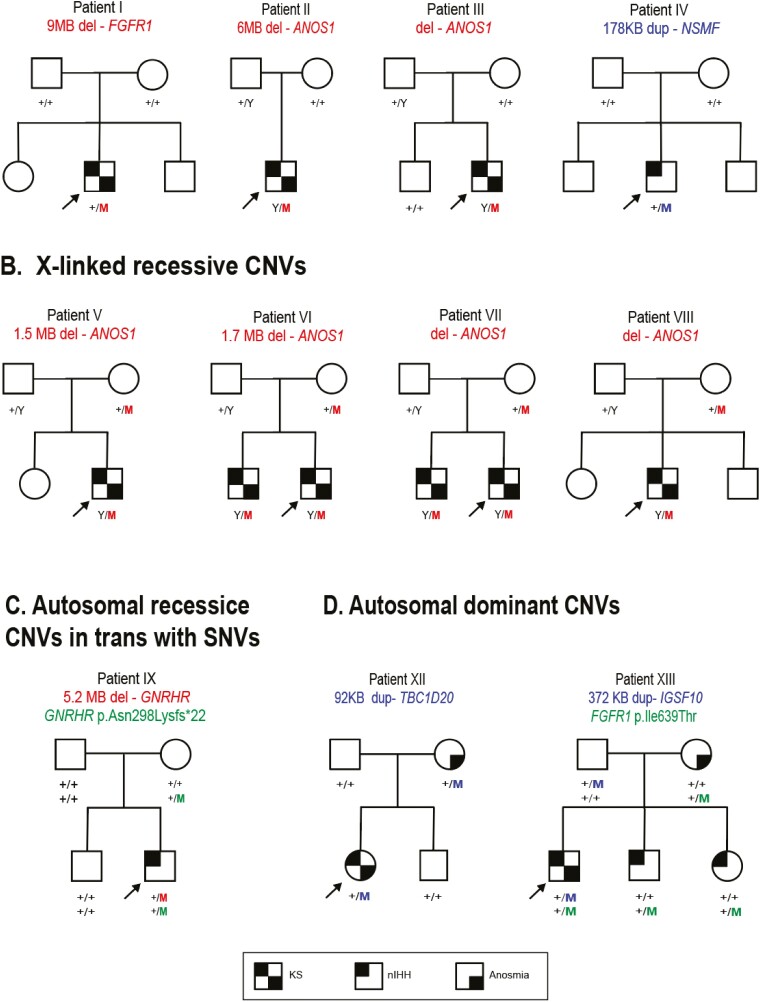
Segregation analysis was feasible in 11 of the IHH pedigrees. This analysis revealed 4 de novo CNVs including 1 multigenic FGFR1 deletion, 2 ANOS1 deletions (multigenic and intragenic) and 1 multigenic NSMF duplication (Fig. 2; panel A). Seven CNVs were inherited with either X-linked, autosomal recessive, or autosomal dominant modes of inheritance. Four ANOS1 deletions were inherited through X-linked transmission (Fig. 2, panel B). One GNRHR multigenic deletion was inherited in trans with a frameshifting SNV in the same gene (p.Asn298Lysfs*22) occurring on the other allele (Fig. 2, panel C). Notably, prior to CNV analysis, this frameshifting SNV was spuriously assigned as being homozygous by ES. While the GNRHR SNV was inherited from an unaffected mother, the large GNRHR deletion occurred de novo on the second allele of the IHH proband. Two homozygous deletions affected the autosomal recessive genes of GNRHR and GNRH1, but parental DNA was not available for familial analysis. Finally, 2 duplications were inherited with an autosomal dominant pattern with incomplete penetrance (Fig. 2, panel D): a proband with KS inherited a duplication spanning TBC1D20 from a mother with anosmia and normal reproductive history and a KS proband inherited a IGSF10 duplication from an unaffected mother.
Figure 2.
Pedigrees with rare CNVs in IHH genes. Panel A: Pedigrees with de novo CNVs; Panel B: Pedigrees with X-linked recessive CNVs; Panel C: Pedigrees with autosomal recessive CNVs and CNVs in trans with SNVs; and Panel D: Pedigrees with autosomal dominant CNVs. Probands are identified by arrows; “+” indicated Wild-type (WT) and allele M indicated the mutant allele. Abbreviations: CNV, copy number variant; IHH, isolated hypogonadotropic hypogonadism; KS, Kallmann syndrome (IHH with anosmia); nIHH, normosmic IHH; SNV, single nucleotide variant.
Genetic Architecture of IHH: Relative and Synergistic Contributions of CNVs and SNVs/Indels
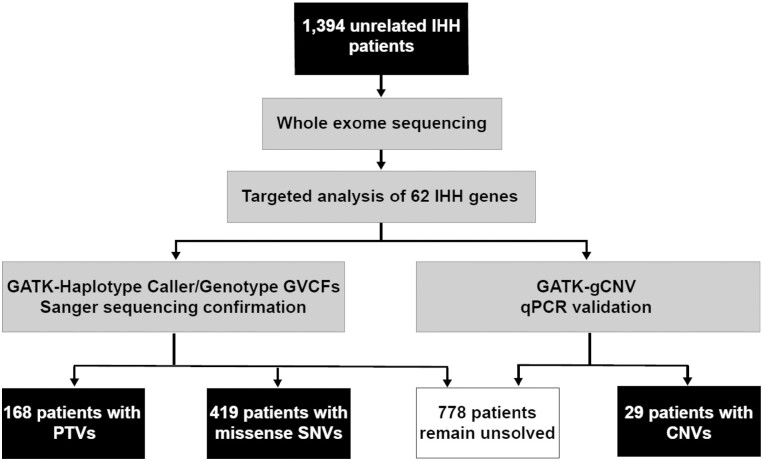
The prevalence of CNVs in IHH probands previously thought to be genetically “solved” was compared with those who were thought to be “unsolved.” The purpose of this comparison was to determine whether the newly identified CNVs acted independently from or synergistically with SNVs/indels. To enable this comparison, ES data was reviewed to extract SNV/indel data on all IHH genes and causative variants were ascertained to determine cases deemed as “solved” (see “Methods”). Using these criteria, 40% of the probands (547/1394) were deemed “solved” [12% with protein truncating variants (PTVs: nonsense, frameshift, and essential splice site) and 28% with missense SNVs]. The remaining 847/1394 (60%) of cases were considered “unsolved” (Fig. 3). The majority of the confirmed CNVs (28/29) were observed in “unsolved” cases, that either lacked SNVs/indels in IHH-associated genes or harbored variants that were inadequate to be solely causal (see “Methods” and Supplemental Table 5 (17)). Thus, the prevalence of CNVs was slightly higher when considering unsolved IHH cases (28/847, 3.4%). Only 1 proband harbored a multigenic IGSF10 duplication that occurred synergistically with a novel heterozygous FGFR1 p.Ile639Thr SNV that was sufficient to be considered a sole causal variant. This observation suggests that the IGSF10 duplication may be coincidental or may contribute to IHH in synergy with the FGFR1 variant through oligogenic mechanisms (Fig. 2, Pedigree XI).
Figure 3.
Flowchart of analysis strategy for the IHH cohort. In this study, 1394 unrelated IHH patients underwent targeted exome sequencing (ES) and subsequent analysis for potentially causal genetic variants in IHH genes. Abbreviations: CNV, copy number variant; IHH, isolated hypogonadotropic hypogonadism; PTV: protein truncating variant; SNV, single nucleotide variant.
The distribution of deletions, duplications, and SNVs/indels differed among the IHH genes (Supplemental Table 6 (17)). ANOS1 was the only gene affected by both deletions and duplications, consistent with the observation that the ANOS1 gene is located within a region prone to nonallelic homology recombination (NAHR) (Supplemental Table 7 (17)/Supplemental References (17)). While ANOS1 and FGFR1 carried a high proportion of both SNVs/indels and CNVs, some genes (eg, CHD7) with a notable SNV/indel contribution lacked any CNVs. The precise basis of lack of CHD7 CNVs is unclear but is in keeping with prior reported CHD7 related CNV prevalence in CHARGE syndrome (20).
Phenotypic Presentation of IHH Probands Carrying CNVs
Multigenic and single genic CNVs in IHH genes resulted in IHH (with or without anosmia) in keeping with their known association with reproductive dysfunction. Review of the nonreproductive phenotypic spectrum revealed that 15/18 subjects with multigenic CNVs demonstrated additional nonreproductive phenotypes (Table 3). All patients with multigenic ANOS1 deletions exhibited syndromic contiguous gene phenotypes that have been previously well-documented (3, 4, 7, 10, 21-68) (Table 3 and Table 4). Interestingly, a KS male patient (Table 3, case 3) with a contiguous gene deletion that only partially deleted the STS gene (the breakpoint occurred within the second intron of this 4-exon gene) did not display ichthyosis, in contrast to all other KS males who harbored deletions spanning the entire STS genomic region (Table 3, Cases 1-2, 4-5). This partial STS deletion may explain the absence of ichthyosis as part of this patient’s phenotypic presentation. Two additional KS males harbored larger Xp22.31 deletions spanning the genes of ANOS1, STS, ARSD, and ARSF (Table 3, Cases 4 and 5), and, not surprisingly, displayed KS, ichthyosis, and chondrodysplasia (3, 4, 25). They also demonstrated learning disabilities. Rare Xp22.31 deletions and mutations in the NLGN4X and VCX genes that reside within the Xp22.31 region have been previously linked to intellectual disability and thus, deletion of those genes may attribute to the subjects’ complex phenotypic presentations (69, 70).
Table 3.
Phenotypic characteristics of IHH probands with rare CNVs affecting IHH genes
| Case | CNV | No. of genes | IHH gene | Gender | Primary diagnosis | Additional phenotypic features |
|---|---|---|---|---|---|---|
| Multigenic deletions | ||||||
| 1 | Deletion | 7 | ANOS1 | M | KS | kidney abnormalities, synkinesia, ichthyosis |
| 2 | Deletion | 7 | ANOS1 | M | KS | renal agenesis, synkinesia, ichthyosis |
| 3 | Deletion | 6 | ANOS1 | M | KS | synkinesia, renal agenesis |
| 4 | Deletion | 20 | ANOS1 | M | KS | clinodactyly, scoliosis, excessive joint motility, flattened nose bridge, gap between teeth, hearing loss, learning disabilities, MR, seizures, other neuro, ichthyosis |
| 5 | Deletion | 16 | ANOS1 | M | KS | syndactyly, hypotelorism, strabismus, flattened nose bridge, learning disability, synkinesia, ichthyosis |
| 6 | Deletion | 49 | FGFR1 | M | KS | strabismus, missing teeth, hearing loss |
| 7 | Deletion | 40 | FGFR1 | M | KS | spherocytosis, bent digits, color blindness, other eye disorders, cleft lip/palate, crowded teeth, double uvula, face/mouth surgery, missing teeth, peripheral neuropathy, speech impairment |
| 8 | Deletion | 25 | FGFR1 | M | KS | alopecia |
| 9 | Deletion | 33 | FGFR1 | M | KS | short 4th metacarpals |
| 10 | Deletion | 26 | FGFR1 | M | nIHH | none |
| 11 | Deletion | 20 | GNRHR | M | nIHH | strabismus |
| 12 | Deletion | 49 | WDR11 | F | KS | hearing loss, clinodactyly, learning disability |
| 13 | Deletion | 28 | NDNF | M | nIHH | none |
| Single genic deletions (full gene, intragenic, and partial) | ||||||
| 14 | Deletion | 1 | GNRH1 | M | nIHH | pectus excavatum, foreshortened arm/leg, excessive joint mobility |
| 15 | Deletion | 1 | SEMA3A | M | KS | learning disability, pes cavus, flat feet |
| 16 | Deletion | 1 | GNRHR | M | nIHH | None |
| 17 | Deletion | 1 | ANOS1 | M | KS | syndactyly, ADHD |
| 18 | Deletion | 1 | ANOS1 | M | KS | Pyloric stenosis |
| 19 | Deletion | 1 | ANOS1 | M | KS | None |
| 20 | Deletion | 1 | ANOS1 | M | KS | None |
| Multigenic duplications | ||||||
| 21 | Duplication | 5 | NSMF | M | KS | synkinesia, scoliosis, bone abnormalities |
| 22 | Duplication | 7 | IGSF10 | M | nIHH | hypotelorism, nystagmus, crowded teeth |
| 23 | Duplication | 4 | KLB | M | nIHH | polydactyly, deviated septum, hearing loss, peripheral neuropathy |
| 24 | Duplication | 3 | TBC1D20 | M | KS | nystagmus, ophthalmoplegia, ptosis, speech impairment, cerebral ataxia |
| 25 | Duplication | 3 | PROP1 | F | nIHH | None |
| Single genic duplications (full gene, intragenic, and partial) | ||||||
| 26 | Duplication | 1 | ANOS1 | M | KS | ataxia, pectus excavatum, crowded teeth, arched broad eyebrows, learning disability, synkinesia |
| 27 | Duplication | 1 | ANOS1 | M | nIHH | None |
| 28 | Duplication | 1 | ANOS1 | F | KS | None |
| 29 | Duplication | 1 | PROKR2 | M | KS | cleft lip and palate |
Abbreviations: CNV, copy number variant; IHH, isolated hypogonadotropic hypogonadism; KS, Kallmann syndrome (ie, IHH with anosmia); nIHH, normosmic IHH.
Table 4.
Deletions spanning the ANOS1 genomic region and associated phenotypes
| Deletion | Phenotype | CDS | Reference |
|---|---|---|---|
| chrXp deletion | KS and ichthyosis | Yes | Ballabio et al; Hum Genet. (1986) (3) |
| chrXp22.3-ter deletion | KS and ichthyosis | Yes | Ballabio et al; Genomics. (1989) (21) |
| chrXp22.31 deletion | Ichthyosis, KS, and CDP | Yes | Bick et al; Am J Med Genet. (1989) (4) |
| chr Xp22-pter deletion | Short stature, CDP, DD, STS deficiency, and KS | Yes | Ballabio et al; PNAS. (1989) (22) |
| chrXp22.31 deletion | KS, STS deficiency, and CDP | Yes | Bick et al; Prenat Diagn. (1992) (23) |
| Intragenic ANOS1 deletion | KS | No | Bick et al; N Engl J Med. (1992) (24) |
| 46X,+der(X),t(X;Y)(p22.31;q11.21), Y | short stature, DD, nasal hypoplasia, telebrachydactyly, hypoplastic genitalia, CDP, ichthyosis | Yes | Wulfsberg et al; Am J Med Genet. (1992) (25) |
| chrXp22.32 deletion | KS, STS deficiency, and URA | Yes | Bouloux et al; Clin Genet. (1993) (26) |
| ANOS1 deletion | KS, URA, synkinesia, unilateral absence of vas deferens, and sensory neural hearing loss | No | Hardelin et al; J Clin Endocrinol Metab. (1993) (27) |
| chrXp22.3 deletion | KS, CDP, short stature, ichthyosis, DD | Yes | Meindl et al; J Med Genet. (1993) (28) |
| chrXp22.3 deletion | KS, ichthyosis, and DD | Yes | Klink et al; Hum Genet, (1994) (29) |
| chrXp22.I-pter deletion | KS, ichthyosis, DD, CDP, and short stature | Yes | Paige et al; Br J Dermatol. (1994) (30) |
| chrXp22.3 deletion | KS, ichthyosis, and URA | Yes | Martul et al, Clin Endocrinol. (1995) (31) |
| chrXp22.3 deletion (STS and partial ANOS1 deletion) | KS and ichthyosis | Yes | Parenti et al; Am J Med Genet. (1995) (32) |
| chrXpter deletion (ANOS1 and STS) | Synkinesia, ichthyosis, URA, diastolic hypertension, renal impairment, proteinuria, cryptorchidism, micropenis, and hypothyroidism | Yes | Quinton et al; J Clin Endocrinol Metab. (1996) (33) |
| ANOS1 deletion | Synkinesia, URA, and cryptorchidism | No | Quinton et al; J Clin Endocrinol Metab. (1996) (33) |
| ANOS1 deletion (exon 1-11) | Synkinesia, cryptorchidism, and Marfanoid habitus | No | Quinton et al; J Clin Endocrinol Metab. (1996) (33) |
| ANOS1 deletion (exon 1-11) | Exon 1 deleted Exon 11 deleted BS, cryptorchid Marfanoid habitus, cryptorchidism | No | Quinton et al; J Clin Endocrinol Metab. (1996) (33) |
| ChrXp22.3 interstitial deletion | Short stature, DD, OA, foveal hypoplasia. ichthyosis, horizontal nystagmus, micropenis, hypoplastic scrotum, bilateral cryptorchidism, and lack of pubic hair | Yes | Muroya et al; Am J Med Genet. (1996) (34) |
| X-Y chromosomal translocation involving the Xp22.3 locus (STS deletion and ANOS1 exons 10-14 deletion) | KS and ichthyosis | Yes | Quinton et al; Clin Exp Dermatol. (1997) (35) |
| ANOS1 (exons 1-3) and STS deletion | KS and ichthyosis | Yes | Maya-Nunez et al; Clin Endocrinol. (1998) (36) |
| chrXp22.3 deletion | KS, ichthyosis, and DD | Yes | Weissortel et al; Clin Genet. (1998) (37) |
| ANOS1 deletion | KS and URA | No | Zenteno et al; BJU Int. (1999) (38) |
| chrXp22.3 deletion | ichthyosis, bilateral cryptorchidism, hyposmia, synkinesia, and DD | Yes | Hou et at; J Formos Med Assoc. (1999) (39) |
| ANOS1 deletion | Micropenis and cryptorchidism | No | Hou et al; J Formos Med Assoc. (1999) (39) |
| ANOS1 deletion (exons 5-10) | KS and URA | No | Nagata et al; J Hum Genet. (2000) (40) |
| ANOS1 deletion (exon 5) | KS, pes cavus, and facial asymmetry | No | Soderlund et al; J Clin Endocrinol Metab. (2002) (41) |
| ANOS1 deletion (exons 3-13) | KS, URA, high-arched palate, brachymetacarpia, hearing loss, synkinesia, and abnormal eye movements | No | Massin et al; J Clin Endocrinol Metab. (2003) (42) |
| chrXp22.3 deletion | KS, synkinesia, URA, DD, ichthyosis, and OA | Yes | Sato et al; J Clin Endocrinol Metab. (2004) (43) |
| chrXp22.3 deletion | KS, DD, ichthyosis, and OA | Yes | Sato et al; J Clin Endocrinol Metab. (2004) (43) |
| ANOS1 deletion (exons 5–14) | KS, URA, and DD | No | Trarbach et al; Genet Mol Biol. (2004) (44) |
| ANOS1 deletion (exons 5–10) | KS, RA, synkinesia, high-arched palate | No | Trarbach et al; Genet Mol Biol. (2004) (44) |
| ChrXp22.3 deletion | KS, ichthyosis, DD | Yes | Hou et al; Chang Gung Med J. (2005) (45) |
| ChrXp22.3 deletion | KS, ichthyosis, DD, and OA | Yes | Hou et al; Chang Gung Med J. (2005) (45) |
| ChrXp22.3 deletion | KS, ichthyosis, DD, OA, DD, and CDP | Yes | Hou et al; Chang Gung Med J. (2005) (45) |
| ANOS1 deletion | Complete deletion of KAL-1 locus, high-arched palate sporadic | No | Trarbach et al; J Endocrinol. (2005) (46) |
| ANOS1 deletion (exons 5-10) | KS, renal agenesis, DD | No | Trarbach et al; J Endocrinol. (2005) (46) |
| ANOS1 deletion (exons 5-10) | KS, URA, high-arched palate, and synkinesia | No | Trarbach et al; J Endocrinol. (2005) (46) |
| ANOS1 deletion (exons 3-6) | KS | No | Trarbach et al; J Clin Endocrinol Metab. (2006) (47) |
| chrXp22.2-22.3 (7.7 Mb) | Congenital nystagmus, retinal dystrophy, strabismus, broad depressed nasal bridge, low set ears, clinodactyly of the fifth finger, left-sided cryptorchidism with absence of the right testis, ichthyosis, and DD | Yes | Chocholska et al; Am J Med Genet. (2006) (48) |
| ChrXp22.31 46,Y,del(X)(p22.31) (9.6 MB) | Multiple minor facial anomalies, small nose with narrow nares, short fingers with brachytelephalangy, unilateral simian crease, cryptorchidism, ichthyosis, bowing of legs, mild aortic valve insufficiency, bilateral sensorineural deafness, OA, bilateral nystagmus, and psychomotor delay | Yes | Melichar et al; Am J Med Genet. (2007) (49) |
| chrXp22.3 (4.5Mb) | KS, ichthyosis, and mild ID | Yes | Macarov et al; J Intellect Diasabil Res. (2007) (50) |
| Inverted duplication of the Xp22.31-Xp22.32 (13.7 Mb)/ terminal Xp deletion Xp22.33-Xpter | Small stature, Madelung deformity, facial dysmorphism, mild DD, and behavioral problems | Yes | Dupont et al; Am J Med Genet. (2007) (51) |
| chrXp22.3 deletion (7Mb) | KS and ichthyosis | Yes | Mochel et al; Eur J Med Genet. (2008) (52) |
| chrXp22.3 (Multigenic ANOS1 deletion) | KS, synkinesia, and URA | Yes | Hershkovitz et al; Horm Res. (2008) (53) |
| ANOS1 deletion | KS | No | Pedersen White et al; Mol Hum Reprod. (2008) (7) |
| ANOS1 deletion (exon 4) | KS and URA | No | Pedersen White et al; Mol Hum Reprod. (2008) (7) |
| ANOS1 deletion | KS and visual field abnormality | No | Pedersen White et al; Mol Hum Reprod. (2008) (7) |
| ANOS1 deletion (exon 6) | KS and ASD | No | Tang et al; Asian J Androl. (2009) (54) |
| ANOS1 deletion (exons 5 and 6) | KS | No | Tang et al; Asian J Androl. (2009) (54) |
| ANOS1 deletion | KS | No | Krzyminska et al; Endokrynl Pol. (2011) (55) |
| chrXp—Xp22 deletion (9.7 Mb) | KS, CDP, developmental delay, ichthyosis, and OA | Yes | Cho et al; J Korean Med Sci. (2012) (56) |
| chrXp22.3 (2.2 Mb—ANOS1 andGPR143) | KS and OA | Yes | Vasson et al; Eur J Hum Genet. (2013) (57) |
| ANOS1 deletion (exons 1–2) | KS | No | Montenegro et al; Fertil Steril. (2013) (58) |
| ANOS1 deletion (exons 3–14) | KS | No | Montenegro et al; Fertil Steril. (2013) (58) |
| ANOS1 deletion (exon 9, exon 11 duplication) | KS | No | Basaran et al; Endokrynl Pol. (2013) (10) |
| chrXp22.31 deletion (570KB: 8 112 876-8 665 494) | KS and hearing loss | Yes | Marlin et al; Otol Neurotol. (2013) (59) |
| chrXp22.3 (ANOS1 exon 9-14 deletion and STS) | KS and ichthyosis | Yes | Xu et al; Andrologia. (2015) (60) |
| ANOS1 deletion (exons 4 -6) | KS | No | Ahmadzadeh et al; Int J Mol Cell Med. (2015) (61) |
| Xp22.31-p22.33 (5.4Mb) | KS and steroid sulafatase deficiency | Yes | Liu et al; Zhonglua Yi Xue Yi Chuan Xue Za Zhi. (2016) (62) |
| chrXp22.3 deletion (4.8Mb) | KS and ichthyosis | Yes | Goncalves et al; Hum Reprod. (2017) (63) |
| chrXp22.3 deletion (2.7 Mb) | KS and ichthyosis | Yes | Nagai et al; Cytogenet Genome Res. (2017) (64) |
| chrXp22.3 deletion (0.24 Mb, chrX:8536480—chrX:8730416) | KS | Yes | Niu et al; Androologia. (2018) (65) |
| chrXp22.3 deletion | KS and ichthyosis | Yes | Berges-Raso et al; Endocrinol Diabetes Metab Case Rep. (2017) (66) |
| chr Xp22.31 region (3,9 Mb, chrX: 5810838-9733877) | KS, ichthyosis, obesity, hyperlipidemia, and strabismus | Yes | Ma et al; Front Genet. (2020) (67) |
| 46,Xp+,Y, 8.3 Mb deletion of Xp22.33 (including 7-14 ANOS1 exon deletion) and 11.22 Yq11.22 duplication | Delayed secondary sexual characteristics, impaired sense of smell, and poor scholastic performance | Yes | Sait et al; J Reprod Infertil. (2021) (68) |
Reported cases with deletions spanning the gene of ANOS1 (full gene, intragenic and contiguous gene deletions syndromes [CDS] with associated phenotypes).
Abbreviations: ASD, atrial septal defect; CDP, chondrodysplasia punctata; DD, developmental delay; GD, growth delay; KS, Kallmann syndrome (ie, isolated hypogonadotropic hypogonadism with anosmia); MVR, mitral valve regurgitation; nIHH, normosmic isolated hypogonadotropic hypogonadism; OA, ocular albinism; PDA, patent ductus arteriosus; STS, steroid sulfatase; URA, unilateral renal agenesis.
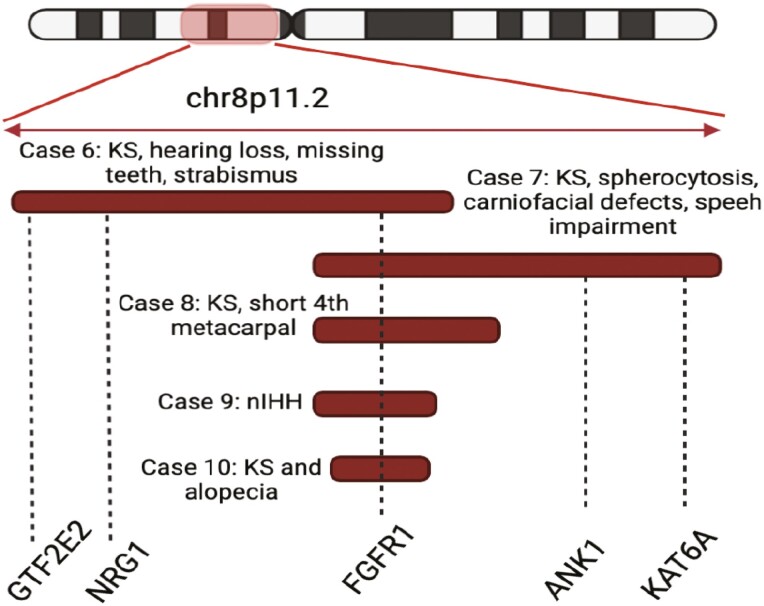
Five probands with multigenic deletions spanning 24 to 47 genes, including FGFR1, were also identified (Table 3, Cases 6-10). Among these subjects, 4 out of the 5 IHH probands displayed additional nonreproductive features, including bone abnormalities, eye defects, craniofacial defects, hearing loss, alopecia, and neurologic abnormalities, including speech impairment. Case 7 harbored a large multigenic deletion of chr8p11.2 spanning the genes of FGFR1 and ANK1 resulting in KS and spherocytosis, an association that has also been well-documented (Table 3 and Table 5) (5, 8, 71-78). Apart from spherocytosis, this KS subject demonstrated craniofacial defects and speech impairment. Review of the deleted genomic region revealed that the KAT6A gene that resides within the chr8p11.2 was affected by the CNV in this KS subject but was spared in all other KS subjects with chr8p11.2 deletions who demonstrated no such neurodevelopmental defects (Table 3/Fig. 4, Cases 6, 8-10). KAT6A has been previously associated with craniofacial defects and speech impairment, and thus its deletion is likely the cause of the additional nonreproductive features in this subject (79). Furthermore, a KS male with hearing loss was found to harbor a FGFR1 multigenic deletion spanning 2 genes (NRG1 and GTF2E2) that have previously been associated with hearing loss (80-82) (Table 3/Fig. 4, Case 6).
Table 5.
Deletions Spanning the FGFR1 Genomic Region and Associated Phenotypes
| Deletion | Phenotype | CDS | Reference |
|---|---|---|---|
| chr8p11.2p21.1 deletion | Cryptorchidism, micropenis, spherocytosis, GD, DD, microcephaly, micrognathia, high-arched palate, epicanthal folds, hypoplastic nails, sacral dimple | Yes | Kitakani et al; Hum Genet. (1988) (71) |
| chr8p11.2p21.1 deletion | Cryptorchidism, spherocytosis, GD, DD, bilateral strabismus, nystagmus, HD | Yes | Lux et al; Nature. (1990) (72) |
| chr8p11.2p21.1 deletion | Failure of sexual development, spherocytosis, GD, DD, microcephaly, micrognathia, bilateral conductive hearing loss, bat ears, bilateral shortened Achilles tendons | Yes | Cohen et al; BJH Int. (1991) (73) |
| chr8p11.2p21.1 deletion | Failure of sexual development, undetectable gonadotropins, spherocytosis, GD, DD, microcephaly, micrognathia, bilateral bat ears, torticollis, fusion of several vertebrae, DMII | Yes | Cohen et al; BJH Int. (1991) (73) |
| chr8p11.2p21.1 deletion | Bilateral cryptorchidism, micropenis, spherocytosis, cleft lip and palate | Yes | Stratton et al; Am J Med Genet. (1992) (74) |
| chr8p11.2p21.1 deletion | Bilateral cryptorchidism, micropenis spherocytosis, GD, DD, microcornea, ASD, PDA, MVR, bone abnormalities, retinal dysplasia | Yes | Okamoto et al; Am J Med Genet. (1995) (75) |
| Chr8p11.1p12 deletion | Anosmia, bilateral cryptorchidism, micropenis, spherocytosis, GD, and mild DD | Yes | Cau et al; Am J Med Genet A. (2005) (76) |
| Chr8p11.2 deletion (FGR1 and ANK1) | KS, spherocytosis, GD, micrognathia, and bilateral crumpled ears | Yes | Vermeulen et al; Am J Med Genet. (2002) (5) |
| FGFR1 deletion | nIHH and ogival palate and cavus foot | No | Trarbach et al; Clin Endocrinol. (2010) (8) |
| Chr8-11.22-12 deletion (8.5 Mb, FGFR1, and 56 genes) | Combined pituitary hormone pituitary deficiency, Chiari type I malformation and syringomyelia, DD, and short stature | Yes | Fukami et al; Endocr J. (2013) (77) |
| Chr8-11.22-12 deletion (5.1 Mb, FGFR1, and 29 genes) | nIHH | Yes | Izumi et al; Fertil Steril. (2014) (78) |
Reported cases with deletions spanning the gene of FGFR1 [full gene, intragenic and contiguous gene deletions syndromes (CDS)] with associated phenotypes.
Abbreviations: ASD, atrial septal defect; DD, developmental delay; GD, growth delay; HD, Hirschsprung’s disease; KS, Kallmann syndrome (ie, isolated hypogonadotropic hypogonadism with anosmia); MVR, mitral valve regurgitation; nIHH, normosmic isolated hypogonadotropic hypogonadism; PDA, patent ductus arteriosus.
Figure 4.
Five IHH subjects harbored multigenic deletions spanning the FGFR1 gene, as well as other chr8p11.2 genes that provide additional genotype-phenotype correlations: the ANK1 gene is linked to spherocytosis, the KAT46A with speech impairment and NGR1 and GTF2E2 with hearing loss, observed in a subset of IHH subjects (figure created with biorender.com).
In addition to IHH subjects with multigenic CNVs, 7/11 subjects with single IHH gene CNVs involving 4 genes (ANOS1, GNRH1, SEMA3A, and PROKR2) also demonstrated additional complex nonreproductive features (Table 3). The nonreproductive phenotypes associated with the CNVs in each of the IHH genes varied: the GNRH1 deletion was associated with bone abnormalities; the ANOS1 deletion with syndactyly; the SEMA3A deletion with learning disability; the ANOS1 intragenic duplication with ataxia, synkinesia, learning disability and bone abnormalities; and the PROKR2 duplication with cleft lip/palate. These data suggest potential pleiotropy of the IHH genes or disruption of putative regulatory elements by the CNVs that may remotely regulate other genes.
Discussion
The CNV Contribution to the Genetic Architecture of IHH
Although CNVs and genomic rearrangements have contributed to important IHH gene discoveries (3-6), targeted CNV analyses in IHH genes had previously been limited to a subset of genes, and/or conducted using low-resolution CNV capturing tools (8, 9). This study used a large patient cohort and a novel, well validated, high-resolution CNV capture technology and identified an overall CNV prevalence of 2% that affects 13/62 known IHH genes. These findings support the notion that CNVs in known IHH genes should be sought out through molecular testing in IHH patients, but they only contribute to a minor proportion of the missing heritability of IHH. Importantly, the high confirmation rate of the reported CNVs highlights the high accuracy of GATK-gCNV pipeline in calling CNVs from ES data. Given that ES can be used for detecting both SNVs/indels and CNVs affecting the coding genome, these findings also strongly support incorporation of ES-based approaches in research and clinical genetic screening.
The CNV prevalence in IHH is in keeping with CNV architecture in similar Mendelian disorders. In a recent study of 1500 Mendelian genes, only 384 genes harbored pathogenic CNVs (83). While some disorders (eg, pediatric/neurologic disorders) were highly enriched for CNVs, other disorders (eg, familial cardiomyopathy) did not have a significant CNV contribution, similar to our observations for IHH (83). The current study suggests that the larger proportion of the missing heritability in IHH may relate to novel coding or noncoding genes that have not yet been discovered. As the use of genome sequencing is expanding, these elusive variants affecting noncoding regions will help reveal the full genetic architecture of IHH.
Pathogenic Mechanisms Underlying Detected CNVs
In this study, rare CNVs (both deletions and duplications) were enriched in the IHH cohort compared with controls. All deletions (multigenic and single gene) were deemed pathogenic by the American College of Medical Genetics and Genomics recommendations (19), and they likely resulted in IHH through loss-of-function (LoF) mechanisms. In addition to the deletions, all duplications were either novel or occurred at very low frequency in controls. Three duplications were intragenic and 6 spanned the full genomic region of the IHH gene. It has been previously shown that intragenic duplications may affect highly constrained genes and lead to LoF by disrupting the coding sequence of the gene (Supplemental References (17)). However, the mechanisms by which duplications spanning the full length of the gene (as seen in 6/9 duplications of this study) result in the IHH phenotype remain uncertain. One possible mechanism is that genes may be sensitive to dosage gain (Supplemental References (17)) and thus, full gene duplications may lead to altered gene expression. In addition, duplications of regulatory regions upstream and downstream of the coding region could affect the expression of the target gene. The mechanisms by which full gene duplications cause phenotypic expression warrant additional studies.
Integrative Analysis of CNVs and SNVs/Indels in IHH Genes
By juxtaposing the CNV analyses with SNV/indel data in known IHH genes, the independent contribution of CNVs to IHH etiology and the combinatorial SNV/CNV contribution was further examined. The vast majority of identified CNVs occurred in IHH cases that were deemed genetically “unsolved” (unsolved cases CNV prevalence: ~3.4%). This observation suggests that CNVs may contribute to IHH pathogenesis independently or in synergy with other variants that were not solely sufficient to cause IHH. Furthermore, this finding also suggests that future novel gene discovery efforts in IHH should incorporate CNV analyses. Previous studies have shown that in some instances, CNVs may also occur in trans with SNVs/indels resulting in recessive forms of Mendelian diseases (84). In keeping with this observation, in this study, segregation analysis of CNVs with SNVs/indels showed that 1 IHH individual carried a de novo multigenic GNRHR deletion on 1 allele and an inherited frameshift SNV on the other allele of the same gene (Fig. 2, Panel C). Despite the rarity of compound heterozygous CNVs and SNVs/indels, such observations are important to document. In the absence of CNV analyses, this SNV appeared to be homozygous and without CNV analyses, this could possibly lead to incorrect genetic counseling. This is particularly important since IHH patients can be successfully treated to induce fertility and erroneous counseling may have implications on pregnancy planning/prenatal genetics.
Comparative Analysis of the Contribution of CNVs and SNVs/Indels in Known IHH Genes
For this comparative analysis, 3 IHH genes with the highest burden of SNVs/indels (FGFR1, CHD7, and ANOS1, Supplemental Table 6 (17)) were compared to their respective CNV burden. Both FGFR1 and ANOS1 demonstrated a high burden of CNVs. Notably, ANOS1 was the only gene affected by both deletions and duplications. These observations are in line with the fact that ANOS1 resides within nonallelic homology recombination (NAHR) regions (Supplemental Table 7 (17)/Supplemental References (17)) that can also explain the prevalence of recurrent ANOS1 related contiguous gene deletion syndromes (3, 4, 63, 64, 66). In contrast, the CHD7 gene was not affected by CNVs. Hitherto, CHD7 mutations have been linked to the severe CHARGE syndrome as well as to IHH with/without CHARGE features (85). These causal CHD7 alleles are predominantly SNVs (85) and CHD7 CNVs contribute to a very small proportion (< 5%) of CHARGE syndrome cases (20) and more likely in those with severe CHARGE syndrome (86-88). Given that the ascertainment of this study was based on IHH, the lack of CHD7 CNVs may relate to the lack of severe CHARGE patients in this study cohort.
Phenotypic Effects of CNVs in IHH
In this study, the detection of multigenic CNVs spanning ANOS1 and FGFR1 confirmed previously suspected contiguous gene deletion syndromes (Tables 4 and 5). Furthermore, our analysis revealed important genotype-phenotype associations. When the contiguous gene deletions spanning the FGFR1 gene were examined, a few genes appeared to be associated with additional nonreproductive features. A KS male subject with craniofacial defects and speech impairment harbored a large deletion spanning the KAT6A gene, which resides within chr8p11.2. KAT6A is linked to the Arboleda-Tham syndrome, an autosomal dominant disorder characterized by intellectual disability, speech delay, microcephaly, cardiac anomalies, and gastrointestinal complications (79). In addition, a single case of KS and mental retardation with a chr8p11.2 was described recently (89). In contrast, all other IHH cases who harbored deletions spanning FGFR1 but no KAT6A lacked any such neurodevelopmental features. Even though precise genotype-phenotype correlations cannot be made with certainty, given the rarity of the CNVs and the complexity of the phenotypes, these data support the association of KAT6A with craniofacial and neurodevelopmental defects in KS patients with chr8p11.2 deletion syndromes. In addition, a KS subject with a multigenic FGFR1 deletion who demonstrated hearing loss was found to harbor a large deletion spanning 2 genes previously implicated in hearing loss: NGR1 has been linked to hearing loss both in genome-wide association and in vivo studies (80, 81) and GTF2E2 mutations have been described in patients with trichothiodystrophy, a multisystem developmental disorder that is characterized by multiple features including bilateral sensorineural hearing loss (82).
Finally, in addition to subjects with multigenic CNVs, 63% of the IHH subjects with single gene CNVs presented with additional nonreproductive phenotypes, suggesting that individual IHH genes by themselves may participate in developmental pleiotropy. Alternatively, disruption of putative regulatory elements within these monogenic CNVs may affect expression of other remote genes, contributing to syndromic phenotypes. From a clinical genetics standpoint, these observations suggest that comprehensive multiorgan evaluation should be performed in all IHH patients harboring CNVs (both single gene and multigenic).
Beyond CNVs: Other “Missing” Elements in the Understanding of IHH Genetic Architecture
Although this study specifically examined the role of CNVs in the missing heritability of IHH, the methodology employed also addressed an important challenge relating to IHH genetic architecture. An important area of uncertainty in IHH genetics relates to deciphering the precise causal role for several IHH genes/variants specifically when they are identified in the heterozygous state. In this regard, a gene-based burden testing between cases vs controls can help provide statistical population-based evidence for inferring causality for genes/variants. Utilizing the robust size of the IHH study cohort (N = 1394), gene-burden testing was performed for 62 IHH genes between cases and controls (gnomAD database) for SNVs/indels using stringent variant criteria (see “Methods”). As shown in Supplemental Table 2 (17), only a subset of the putatively dominant IHH genes showed enrichment in the heterozygous state, suggesting that such genes may contribute to IHH through a true autosomal dominant mechanism. IHH subjects with variants in genes lacking such enrichment could be considered as those likely to require additional genetic hits (ie, oligogenicity) or other modifiers to cause IHH. These findings relating to genes that show enrichment for heterozygous variants may help both researchers and clinicians to infer causality for specific genes/variants.
Ever since the recognition of the IHH phenotype, a nearly 3-fold male predominance has been observed (90). To date, there is currently no clear genetic explanation for this differential sex prevalence. In this study, the CNV prevalence was higher in males (2.5%) compared with females (1%) across our cohort. However, since CNVs in ANOS1-related X-linked recessive inheritance accounted for a significant number of CNVs in males, we compared the prevalence of CNVs affecting autosomal genes between the 2 sexes and did not notice any significant difference (1.4% vs 0.7%). Hence, thus far, Mendelian genetics pertaining to SNVs and CNVs fail to fully explain this discordance in sex-prevalence. Notably, similar male predominance (or alternatively stated, female protective effect) has been reported for other traits such as autism spectrum disorder (ASD) (91)). It has been shown that genetic liability threshold for ASD may be different between the 2 sexes with greater etiologic genetic load required in females compared to males. Future studies in IHH should examine such liability threshold hypothesis to understand the “missing IHH females.”
Limitations/Future Directions
This study has some limitations. ES-based CNVs analysis only allowed us to examine the coding regions of the genome. Ongoing genome sequencing studies will enable detection of CNVs in noncoding genomic regions. Although intragenic duplications may result in LoF, the precise mechanism by which gene full duplications affect protein expression requires additional evaluation. Stringent variant criteria within specific modes of inheritance were applied to determine SNVs/indels as causative for “solved” cases. Some IHH genes display multiple modes of inheritance patterns and are also known to participate in oligogenic inheritance (92), outside of their initial implicated inheritance mode. To avoid missing any potential oligogenic mechanisms, we have accumulated all SNVs/indels that occurred synergistically with CNVs, despite being deemed noncausative based on the applied criteria (Supplemental Table 5 (17)).
Conclusion
In conclusion, this study represents the most comprehensive evaluation of copy number variant contribution to IHH genetic architecture and their associated phenotypic spectrum. Furthermore, this study provides a compelling rationale that gene discovery efforts in IHH should focus on novel genes in both coding and noncoding parts of the genome to fully establish the genetic basis of IHH.
Acknowledgments
We thank the families for participating in this study and the referring clinicians for their referral to our research studies.
Glossary
Abbreviations
- AAC
alternate allele count
- CNV
copy number variant
- ES
exome sequencing
- GnRH
gonadotropin-releasing hormone
- IHH
isolated hypogonadotropic hypogonadism
- LoF
loss of function
- MAF
minor allele frequency
- MGH
Massachusetts General Hospital
- RAC
reference allele count
- SNV
single nucleotide variant
Contributor Information
Maria I Stamou, Reproductive Endocrine Unit, Massachusetts General Hospital and the Center for Reproductive Medicine, Boston, MA 02141, USA.
Harrison Brand, Center for Genomic Medicine, Massachusetts General Hospital, Boston, MA 02141, USA; Department of Neurology, Massachusetts General Hospital and Harvard Medical School, Boston, MA 02141, USA; Program in Medical and Population Genetics, Broad Institute of MIT and Harvard, Cambridge, MA 02141, USA; Pediatric Surgical Research Laboratories, Massachusetts General Hospital, Boston, MA 02141, USA.
Mei Wang, Reproductive Endocrine Unit, Massachusetts General Hospital and the Center for Reproductive Medicine, Boston, MA 02141, USA.
Isaac Wong, Center for Genomic Medicine, Massachusetts General Hospital, Boston, MA 02141, USA; Department of Neurology, Massachusetts General Hospital and Harvard Medical School, Boston, MA 02141, USA.
Margaret F Lippincott, Reproductive Endocrine Unit, Massachusetts General Hospital and the Center for Reproductive Medicine, Boston, MA 02141, USA.
Lacey Plummer, Reproductive Endocrine Unit, Massachusetts General Hospital and the Center for Reproductive Medicine, Boston, MA 02141, USA.
William F Crowley, Endocrine Division, Massachusetts General Hospital, Boston, MA 02141, USA.
Michael Talkowski, Center for Genomic Medicine, Massachusetts General Hospital, Boston, MA 02141, USA; Department of Neurology, Massachusetts General Hospital and Harvard Medical School, Boston, MA 02141, USA; Program in Medical and Population Genetics, Broad Institute of MIT and Harvard, Cambridge, MA 02141, USA.
Stephanie Seminara, Reproductive Endocrine Unit, Massachusetts General Hospital and the Center for Reproductive Medicine, Boston, MA 02141, USA.
Ravikumar Balasubramanian, Reproductive Endocrine Unit, Massachusetts General Hospital and the Center for Reproductive Medicine, Boston, MA 02141, USA.
Financial Support
This work was supported by the following grants from the Eunice Kennedy Shriver National Institute of Child Health and Development: P50 HD028138 (The MGH Harvard Center for Reproductive Medicine): S.B.S.; R.B.; M.T.; R01 HD096324: R.B.; F32HD108873: M.I.S; and R01 HD043341: S.B.S.
Disclosures
The authors have nothing to declare.
Data Availability
Data and materials will be made available by the authors individually upon request subject to the data sharing plan and consent provided by the study participants.
References
- 1. Stamou MI, Cox KH, Crowley WF Jr. Discovering genes essential to the hypothalamic regulation of human reproduction using a human disease model: adjusting to life in the “-Omics” Era. Endocr Rev. 2015;36(6):603-621. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 2. Bujakowska KM, Fernandez-Godino R, Place E, et al. . Copy-number variation is an important contributor to the genetic causality of inherited retinal degenerations. Genet Med. 2017;19(6):643-651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ballabio A, Parenti G, Tippett P, et al. . X-linked ichthyosis, due to steroid sulphatase deficiency, associated with Kallmann syndrome (hypogonadotropic hypogonadism and anosmia): linkage relationships with Xg and cloned DNA sequences from the distal short arm of the X chromosome. Hum Genet. 1986;72(3):237-240. [DOI] [PubMed] [Google Scholar]
- 4. Bick D, Curry CJ, McGill JR, Schorderet DF, Bux RC, Moore CM. Male infant with ichthyosis, Kallmann syndrome, chondrodysplasia punctata, and an Xp chromosome deletion. Am J Med Genet. 1989;33(1):100-107. [DOI] [PubMed] [Google Scholar]
- 5. Vermeulen S, Messiaen L, Scheir P, De Bie S, Speleman F, De Paepe A. Kallmann syndrome in a patient with congenital spherocytosis and an interstitial 8p11.2 deletion. Am J Med Genet. 2002;108(4):315-318. [DOI] [PubMed] [Google Scholar]
- 6. Young J, Metay C, Bouligand J, et al. . SEMA3A deletion in a family with Kallmann syndrome validates the role of semaphorin 3A in human puberty and olfactory system development. Hum Reprod. 2012;27(5):1460-1465. [DOI] [PubMed] [Google Scholar]
- 7. Pedersen-White JR, Chorich LP, Bick DP, Sherins RJ, Layman LC. The prevalence of intragenic deletions in patients with idiopathic hypogonadotropic hypogonadism and Kallmann syndrome. Mol Hum Reprod. 2008;14(6):367-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Trarbach EB, Teles MG, Costa EM, et al. . Screening of autosomal gene deletions in patients with hypogonadotropic hypogonadism using multiplex ligation-dependent probe amplification: detection of a hemizygosis for the fibroblast growth factor receptor 1. Clin Endocrinol (Oxf) 2010;72(3):371-376. [DOI] [PubMed] [Google Scholar]
- 9. Amato LGL, Montenegro LR, Lerario AM, et al. . New genetic findings in a large cohort of congenital hypogonadotropic hypogonadism. Eur J Endocrinol. 2019;181(2):103-119. [DOI] [PubMed] [Google Scholar]
- 10. Basaran Y, Bolu E, Unal HU, et al. . [Multiplex ligation dependent probe amplification analysis of KAL1, GNRH1, GNRHR, PROK2 and PROKR2 in male patients with idiopathic hypogonadotropic hypogonadism]. Endokrynol Pol. 2013;64(4):285-292. [DOI] [PubMed] [Google Scholar]
- 11. Zampaglione E, Kinde B, Place EM, et al. . Copy-number variation contributes 9% of pathogenicity in the inherited retinal degenerations. Genet Med. 2020;22(6):1079-1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Manning M, Hudgins L, Professional P, Guidelines C. Array-based technology and recommendations for utilization in medical genetics practice for detection of chromosomal abnormalities. Genet Med. 2010;12(11):742-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Collins RL, Brand H, Karczewski KJ, et al. . A structural variation reference for medical and population genetics. Nature 2020;581(7809):444-451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Balasubramanian R, Crowley WF Jr. Isolated GnRH deficiency: a disease model serving as a unique prism into the systems biology of the GnRH neuronal network. Mol Cell Endocrinol. 2011;346(1-2):4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lewkowitz-Shpuntoff HM, Hughes VA, Plummer L, et al. . Olfactory phenotypic spectrum in idiopathic hypogonadotropic hypogonadism: pathophysiological and genetic implications. J Clin Endocrinol Metab. 2012;97(1):E136-E144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Johnston JJ, Lewis KL, Ng D, et al. . Individualized iterative phenotyping for genome-wide analysis of loss-of-function mutations. Am J Hum Genet. 2015;96(6):913-925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Stamou MI, Brand H, Wang M, Lippincott MF, Wong I, Plummer L, et al. . Supplemental material_prevalence and phenotypic effects of copy number variants in isolated hypogonadotropic hypogonadism. figshare. Dataset posted April 21, 2022. 10.6084/m9.figshare.19199747.v3. [DOI] [PMC free article] [PubMed]
- 18. Karczewski KJ, Francioli LC, Tiao G, et al. . The mutational constraint spectrum quantified from variation in 141 456 humans. Nature. 2020;581(7809):434-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Riggs ER, Andersen EF, Cherry AM, et al. . Technical standards for the interpretation and reporting of constitutional copy-number variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics (ACMG) and the Clinical Genome Resource (ClinGen). Genet Med. 2020;22(2):245-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Vuorela P, Ala-Mello S, Saloranta C, et al. . Molecular analysis of the CHD7 gene in CHARGE syndrome: identification of 22 novel mutations and evidence for a low contribution of large CHD7 deletions. Genet Med. 2007;9(10):690-694. [DOI] [PubMed] [Google Scholar]
- 21. Ballabio A, Carrozzo R, Parenti G, et al. . Molecular heterogeneity of steroid sulfatase deficiency: a multicenter study on 57 unrelated patients, at DNA and protein levels. Genomics 1989;4(1): 36-40. [DOI] [PubMed] [Google Scholar]
- 22. Ballabio A, Bardoni B, Carrozzo R, et al. . Contiguous gene syndromes due to deletions in the distal short arm of the human X chromosome. Proc Natl Acad Sci USA. 1989;86(24):10001-10005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bick DP, Schorderet DF, Price PA, et al. . Prenatal diagnosis and investigation of a fetus with chondrodysplasia punctata, ichthyosis, and Kallmann syndrome due to an Xp deletion. Prenat Diagn. 1992;12(1):19-29. [DOI] [PubMed] [Google Scholar]
- 24. Bick D, Franco B, Sherins RJ, et al. . Brief report: intragenic deletion of the KALIG-1 gene in Kallmann’s syndrome. N Engl J Med. 1992;326(26):1752-1755. [DOI] [PubMed] [Google Scholar]
- 25. Wulfsberg EA, Curtis J, Jayne CH. Chondrodysplasia punctata: a boy with X-linked recessive chondrodysplasia punctata due to an inherited X-Y translocation with a current classification of these disorders. Am J Med Genet. 1992;43(5):823-828. [DOI] [PubMed] [Google Scholar]
- 26. Bouloux PM, Kirk J, Munroe P, et al. . Deletion analysis maps ocular albinism proximal to the steroid sulphatase locus. Clin Genet. 1993;43(4):169-173. [DOI] [PubMed] [Google Scholar]
- 27. Hardelin JP, Levilliers J, Young J, et al. . Xp22.3 deletions in isolated familial Kallmann’s syndrome. J Clin Endocrinol Metab. 1993;76(4):827-831. [DOI] [PubMed] [Google Scholar]
- 28. Meindl A, Hosenfeld D, Bruckl W, et al. . Analysis of a terminal Xp22.3 deletion in a patient with six monogenic disorders: implications for the mapping of X linked ocular albinism. J Med Genet. 1993;30(10):838-842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Klink A, Meindl A, Hellebrand H, Rappold GA. A patient with an interstitial deletion in Xp22.3 locates the gene for X-linked recessive chondrodysplasia punctata to within a one megabase interval. Hum Genet. 1994;93(4):463-466. [DOI] [PubMed] [Google Scholar]
- 30. Paige DG, Emilion GG, Bouloux PM, Harper JI. A clinical and genetic study of X-linked recessive ichthyosis and contiguous gene defects. Br J Dermatol. 1994;131(5):622-629. [DOI] [PubMed] [Google Scholar]
- 31. Martul P, Pineda J, Levilliers J, et al. . Hypogonadotrophic hypogonadism with hyposmia, X-linked ichthyosis, and renal malformation syndrome. Clin Endocrinol (Oxf). 1995;42(2):121-128. [DOI] [PubMed] [Google Scholar]
- 32. Parenti G, Rizzolo MG, Ghezzi M, et al. . Variable penetrance of hypogonadism in a sibship with Kallmann syndrome due to a deletion of the KAL gene. Am J Med Genet. 1995;57(3):476-478. [DOI] [PubMed] [Google Scholar]
- 33. Quinton R, Duke VM, de Zoysa PA, et al. . The neuroradiology of Kallmann’s syndrome: a genotypic and phenotypic analysis. J Clin Endocrinol Metab. 1996;81(8):3010-3017. [DOI] [PubMed] [Google Scholar]
- 34. Muroya K, Ogata T, Matsuo N, et al. . Mental retardation in a boy with an interstitial deletion at Xp22.3 involving STS, KAL1, and OA1: implication for the MRX locus. Am J Med Genet. 1996;64(4):583-587. [DOI] [PubMed] [Google Scholar]
- 35. Quinton R, Schofield JK, Duke VM, et al. . X-linked ichthyosis with hypogonadism: not always Kallmann’s syndrome. Clin Exp Dermatol. 1997;22(4):201-204. [PubMed] [Google Scholar]
- 36. Maya-Nunez G, Cuevas-Covarrubias S, Zenteno JC, Ulloa-Aguirre A, Kofman-Alfaro S, Mendez JP. Contiguous gene syndrome due to deletion of the first three exons of the Kallmann gene and complete deletion of the steroid sulphatase gene. Clin Endocrinol (Oxf). 1998;48(6):713-718. [DOI] [PubMed] [Google Scholar]
- 37. Weissortel R, Strom TM, Dorr HG, Rauch A, Meitinger T. Analysis of an interstitial deletion in a patient with Kallmann syndrome, X-linked ichthyosis and mental retardation. Clin Genet. 1998;54(1):45-51. [DOI] [PubMed] [Google Scholar]
- 38. Zenteno JC, Mendez JP, Maya-Nunez G, Ulloa-Aguirre A, Kofman-Alfaro S. Renal abnormalities in patients with Kallmann syndrome. BJU Int. 1999;83(4):383-386. [DOI] [PubMed] [Google Scholar]
- 39. Hou JW, Tsai WY, Wang TR. Detection of KAL-1 gene deletion with fluorescence in situ hybridization. J Formos Med Assoc. 1999;98(6):448-451. [PubMed] [Google Scholar]
- 40. Nagata K, Yamamoto T, Chikumi H, et al. . A novel interstitial deletion of KAL1 in a Japanese family with Kallmann syndrome. J Hum Genet. 2000;45(4):237-240. [DOI] [PubMed] [Google Scholar]
- 41. Soderlund D, Canto P, Mendez JP. Identification of three novel mutations in the KAL1 gene in patients with Kallmann syndrome. J Clin Endocrinol Metab. 2002;87(6):2589-2592. [DOI] [PubMed] [Google Scholar]
- 42. Massin N, Pecheux C, Eloit C, et al. . X chromosome-linked Kallmann syndrome: clinical heterogeneity in three siblings carrying an intragenic deletion of the KAL-1 gene. J Clin Endocrinol Metab. 2003;88(5):2003-2008. [DOI] [PubMed] [Google Scholar]
- 43. Sato N, Katsumata N, Kagami M, et al. . Clinical assessment and mutation analysis of Kallmann syndrome 1 (KAL1) and fibroblast growth factor receptor 1 (FGFR1, or KAL2) in five families and 18 sporadic patients. J Clin Endocrinol Metab. 2004;89(3):1079-1088. [DOI] [PubMed] [Google Scholar]
- 44. Trarbach EB, Monlleo IL, Porciuncula CGG, Fontes MIB, Baptista MM, Hacket C. Similar interstitial deletions of the KAL-1 gene in two Brazilian families with X-linked Kallmann Syndrome. Hum Med Genet. Published online September 1, 2004;27(3). doi:10.1590/S1415-47572004000300006 [Google Scholar]
- 45. Hou JW. Detection of gene deletions in children with chondrodysplasia punctata, ichthyosis, Kallmann syndrome, and ocular albinism by FISH studies. Chang Gung Med J. 2005;28(9):643-650. [PubMed] [Google Scholar]
- 46. Trarbach EB, Baptista MT, Garmes HM, Hackel C. Molecular analysis of KAL-1, GnRH-R, NELF and EBF2 genes in a series of Kallmann syndrome and normosmic hypogonadotropic hypogonadism patients. J Endocrinol. 2005;187(3):361-368. [DOI] [PubMed] [Google Scholar]
- 47. Trarbach EB, Costa EM, Versiani B, et al. . Novel fibroblast growth factor receptor 1 mutations in patients with congenital hypogonadotropic hypogonadism with and without anosmia. J Clin Endocrinol Metab. 2006;91(10):4006-4012. [DOI] [PubMed] [Google Scholar]
- 48. Chocholska S, Rossier E, Barbi G, Kehrer-Sawatzki H. Molecular cytogenetic analysis of a familial interstitial deletion Xp22.2-22.3 with a highly variable phenotype in female carriers. Am J Med Genet A. 2006;140(6):604-610. [DOI] [PubMed] [Google Scholar]
- 49. Melichar VO, Guth S, Hellebrand H, et al. . A male infant with a 9.6 Mb terminal Xp deletion including the OA1 locus: Limit of viability of Xp deletions in males. Am J Med Genet A. 2007;143A(2):135-141. [DOI] [PubMed] [Google Scholar]
- 50. Macarov M, Zeigler M, Newman JP, et al. . Deletions of VCX-A and NLGN4: a variable phenotype including normal intellect. J Intellect Disabil Res. 2007;51(Pt 5):329-333. [DOI] [PubMed] [Google Scholar]
- 51. Dupont C, Lebbar A, Teinturier C, et al. . First reported case of intrachromosomal cryptic inv dup del Xp in a boy with developmental retardation. Am J Med Genet A. 2007;143A(11):1236-1243. [DOI] [PubMed] [Google Scholar]
- 52. Mochel F, Missirian C, Reynaud R, Moncla A. Normal intelligence and social interactions in a male patient despite the deletion of NLGN4X and the VCX genes. Eur J Med Genet. 2008;51(1):68-73. [DOI] [PubMed] [Google Scholar]
- 53. Hershkovitz E, Loewenthal N, Peretz A, Parvari R. Testicular expressed genes are missing in familial X-Linked Kallmann syndrome due to two large different deletions in daughter’s X chromosomes. Horm Res. 2008;69(5):276-283. [DOI] [PubMed] [Google Scholar]
- 54. Tang KF, Wu QF, Zou TJ, Xue W, Wang XY, Xing JP. Molecular analysis of KAL-1 in a series of Kallmann syndrome and normosmic idiopathic hypogonadotropic hypogonadism patients from Northwestern China. Asian J Androl. 2009;11(6):711-715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Krzyminska A, Hilczer M, Hawula W, Ulanska A, Jakubowski L. Large deletion in the KAL1 gene in two related patients with hypogonadotropic hypogonadism: diagnostic usefulness of cytogenetic and molecular methods. Endokrynol Pol. 2011;62(3):224-229. [PubMed] [Google Scholar]
- 56. Cho EH, Kim SY, Kim JK. A case of 9.7 Mb terminal Xp deletion including OA1 locus associated with contiguous gene syndrome. J Korean Med Sci. 2012;27(10):1273-1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Vasson A, Leroux C, Orhant L, et al. . Custom oligonucleotide array-based CGH: a reliable diagnostic tool for detection of exonic copy-number changes in multiple targeted genes. Eur J Hum Genet. 2013;21(9):977-987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Montenegro LR, Silveira LF, Tusset C, et al. . Combined use of multiplex ligation-dependent probe amplification and automatic sequencing for identification of KAL1 defects in patients with Kallmann syndrome. Fertil Steril. 2013;100(3):854-859. [DOI] [PubMed] [Google Scholar]
- 59. Marlin S, Chantot-Bastaraud S, David A, et al. . Discovery of a large deletion of KAL1 in 2 deaf brothers. Otol Neurotol. 2013;34(9):1590-1594. [DOI] [PubMed] [Google Scholar]
- 60. Xu H, Li Z, Wang T, Wang S, Liu J, Wang DW. Novel homozygous deletion of segmental KAL1 and entire STS cause Kallmann syndrome and X-linked ichthyosis in a Chinese family. Andrologia 2015;47(10):1160-1165. [DOI] [PubMed] [Google Scholar]
- 61. Ahmadzadeh A, Ghods E, Mojarrad M, et al. . Study on KAL1 gene mutations in idiopathic hypogonadotropic hypogonadism patients with X-linked recessive inheritance. Int J Mol Cell Med. 2015;4(3):152-159. [PMC free article] [PubMed] [Google Scholar]
- 62. Liu X, Bai N, Kong X. [Genetic analysis of a rare case with Kallman syndrome and steroid sulfatase deficiency]. Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 2016;33(3):349-352. [DOI] [PubMed] [Google Scholar]
- 63. Goncalves CI, Fonseca F, Borges T, Cunha F, Lemos MC. Expanding the genetic spectrum of ANOS1 mutations in patients with congenital hypogonadotropic hypogonadism. Hum Reprod. 2017;32(3):704-711. [DOI] [PubMed] [Google Scholar]
- 64. Nagai K, Shima H, Kamimura M, et al. . Xp22.31 Microdeletion due to microhomology-mediated break-induced replication in a boy with contiguous gene deletion syndrome. Cytogenet Genome Res. 2017;151(1):1-4. [DOI] [PubMed] [Google Scholar]
- 65. Niu Y, Zhou C, Xu H, et al. . Novel interstitial deletion in Xp22.3 in a typical X-linked recessive family with Kallmann syndrome. Andrologia. 2018;50(4):e12961. doi:10.1111/and.12961 [DOI] [PubMed] [Google Scholar]
- 66. Berges-Raso I, Gimenez-Palop O, Gabau E, Capel I, Caixas A, Rigla M. Kallmann syndrome and ichthyosis: a case of contiguous gene deletion syndrome. Endocrinol Diabetes Metab Case Rep. 2017;2017(1):EDM170083. [DOI] [PubMed] [Google Scholar]
- 67. Ma W, Mao J, Wang X, et al. . Novel microdeletion in the X chromosome leads to kallmann syndrome, ichthyosis, obesity, and strabismus. Front Genet. 2020;11:596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Sait H, Srivastava P, Dabadghao P, Phadke SR. Kallmann Syndrome and X-linked ichthyosis caused by translocation between chromosomes X and Y: a case report. J Reprod Infertil. 2021;22(4):302-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Fukami M, Kirsch S, Schiller S, et al. . A member of a gene family on Xp22.3, VCX-A, is deleted in patients with X-linked nonspecific mental retardation. Am J Hum Genet. 2000;67(3):563-573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Jamain S, Quach H, Betancur C, et al. . Mutations of the X-linked genes encoding neuroligins NLGN3 and NLGN4 are associated with autism. Nat Genet. 2003;34(1):27-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Kitatani M, Chiyo H, Ozaki M, Shike S, Miwa S. Localization of the spherocytosis gene to chromosome segment 8p11.22----8p21. Hum Genet. 1988;78(1):94-95. [DOI] [PubMed] [Google Scholar]
- 72. Lux SE, Tse WT, Menninger JC, et al. . Hereditary spherocytosis associated with deletion of human erythrocyte ankyrin gene on chromosome 8. Nature. 1990;345(6277):736-739. [DOI] [PubMed] [Google Scholar]
- 73. Cohen H, Walker H, Delhanty JD, Lucas SB, Huehns ER. Congenital spherocytosis, B19 parvovirus infection and inherited interstitial deletion of the short arm of chromosome 8. Br J Haematol. 1991;78(2):251-257. [DOI] [PubMed] [Google Scholar]
- 74. Stratton RF, Crudo DF, Varela M, Shapira E. Deletion of the proximal short arm of chromosome 8. Am J Med Genet. 1992;42(1):15-18. [DOI] [PubMed] [Google Scholar]
- 75. Okamoto N, Wada Y, Nakamura Y, et al. . Hereditary spherocytic anemia with deletion of the short arm of chromosome 8. Am J Med Genet. 1995;58(3):225-229. [DOI] [PubMed] [Google Scholar]
- 76. Cau M, Congiu R, Origa R, Galanello R, Melis MA, Nucaro AL. New case of contiguous gene syndrome at chromosome 8p11.2p12. Am J Med Genet A. 2005;136(2):221-222. [DOI] [PubMed] [Google Scholar]
- 77. Fukami M, Iso M, Sato N, et al. . Submicroscopic deletion involving the fibroblast growth factor receptor 1 gene in a patient with combined pituitary hormone deficiency. Endocr J. 2013;60(8):1013-1020. [DOI] [PubMed] [Google Scholar]
- 78. Izumi Y, Suzuki E, Kanzaki S, et al. . Genome-wide copy number analysis and systematic mutation screening in 58 patients with hypogonadotropic hypogonadism. Fertil Steril. 2014;102(4):1130-1136.e3. [DOI] [PubMed] [Google Scholar]
- 79. Arboleda VA, Lee H, Dorrani N, et al. . De novo nonsense mutations in KAT6A, a lysine acetyl-transferase gene, cause a syndrome including microcephaly and global developmental delay. Am J Hum Genet. 2015;96(3):498-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Zhang YM, Yang ZD, Yu YF. Effect of neuregulin-1 on the auditory cortex in adult C57BL/6J mice. Iran J Basic Med Sci. 2020;23(3):362-367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Vuckovic D, Mezzavilla M, Cocca M, et al. . Whole-genome sequencing reveals new insights into age-related hearing loss: cumulative effects, pleiotropy and the role of selection. Eur J Hum Genet. 2018;26(8):1167-1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Kuschal C, Botta E, Orioli D, et al. . GTF2E2 mutations destabilize the general transcription factor complex TFIIE in individuals with DNA repair-proficient trichothiodystrophy. Am J Hum Genet. 2016;98(4):627-642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Truty R, Paul J, Kennemer M, et al. . Prevalence and properties of intragenic copy-number variation in Mendelian disease genes. Genet Med. 2019;21(1):114-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Yuan B, Wang L, Liu P, et al. . CNVs cause autosomal recessive genetic diseases with or without involvement of SNV/indels. Genet Med. 2020;22(10):1633-1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Balasubramanian R, Choi JH, Francescatto L, et al. . Functionally compromised CHD7 alleles in patients with isolated GnRH deficiency. Proc Natl Acad Sci USA. 2014;111(50): 17953-17958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Bergman JE, de Wijs I, Jongmans MC, Admiraal RJ, Hoefsloot LH, van Ravenswaaij-Arts CM. Exon copy number alterations of the CHD7 gene are not a major cause of CHARGE and CHARGE-like syndrome. Eur J Med Genet. 2008;51(5):417-425. [DOI] [PubMed] [Google Scholar]
- 87. van Ravenswaaij-Arts CM, Blake K, Hoefsloot L, Verloes A. Clinical utility gene card for: CHARGE syndrome - update 2015. Eur J Hum Genet. 2015;23(11):3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Wincent J, Schulze A, Schoumans J. Detection of CHD7 deletions by MLPA in CHARGE syndrome patients with a less typical phenotype. Eur J Med Genet. 2009;52(4):271-272. [DOI] [PubMed] [Google Scholar]
- 89. Wang D, Lai P. Global retardation and hereditary spherocytosis associated with a novel deletion of chromosome 8p11.21 encompassing KAT6A and ANK1. Eur J Med Genet. 2020;63(12):104082. [DOI] [PubMed] [Google Scholar]
- 90. Laitinen EM, Vaaralahti K, Tommiska J, et al. . Incidence, phenotypic features and molecular genetics of Kallmann syndrome in Finland. Orphanet J Rare Dis. 2011;6:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Robinson EB, Lichtenstein P, Anckarsater H, Happe F, Ronald A. Examining and interpreting the female protective effect against autistic behavior. Proc Natl Acad Sci USA. 2013;110(13): 5258-5262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Sykiotis GP, Plummer L, Hughes VA, et al. . Oligogenic basis of isolated gonadotropin-releasing hormone deficiency. Proc Natl Acad Sci USA. 2010;107(34):15140-15144. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data and materials will be made available by the authors individually upon request subject to the data sharing plan and consent provided by the study participants.