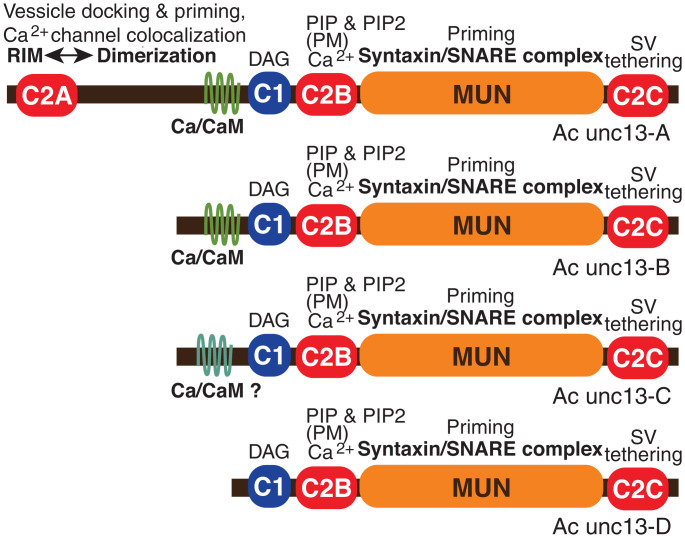
Fig. 5.
Domains in alternative forms of unc13 expressed from alternative start sites from the Aplysia unc13 gene. The heterogeneity of isoforms is similar to the four Munc13 isoforms expressed in mammalian neurons: Munc13-1, bMunc13-2, ubMunc13-2, and Munc13-3. Unc13 interactions illustrated include C2A domain binding to RIM, which relieves inhibitory homodimerization; Ca2+/CaM binding site; C1 domain, which binds DAG; C2B domain, which binds Ca2+ and also binds PIP and PIP2, mediating localization to plasma membrane (PM); MUN domain, which facilitates a critical conformational change in syntaxin and ensures correct assembly of the SNARE complex; and the C terminus, including C2C, which tethers synaptic vesicles (SVs). DAG, Ca2+, and PIP/PIP2 binding to the C1-C2B module triggers activation of the MUN domain (43, 83). Note: Ac unc13-C contains a divergent, putative CaM-binding sequence (SI Appendix, Fig. S14); whether it actually binds Ca2+/CaM is not known (as indicated by “Ca/CaM-?”).