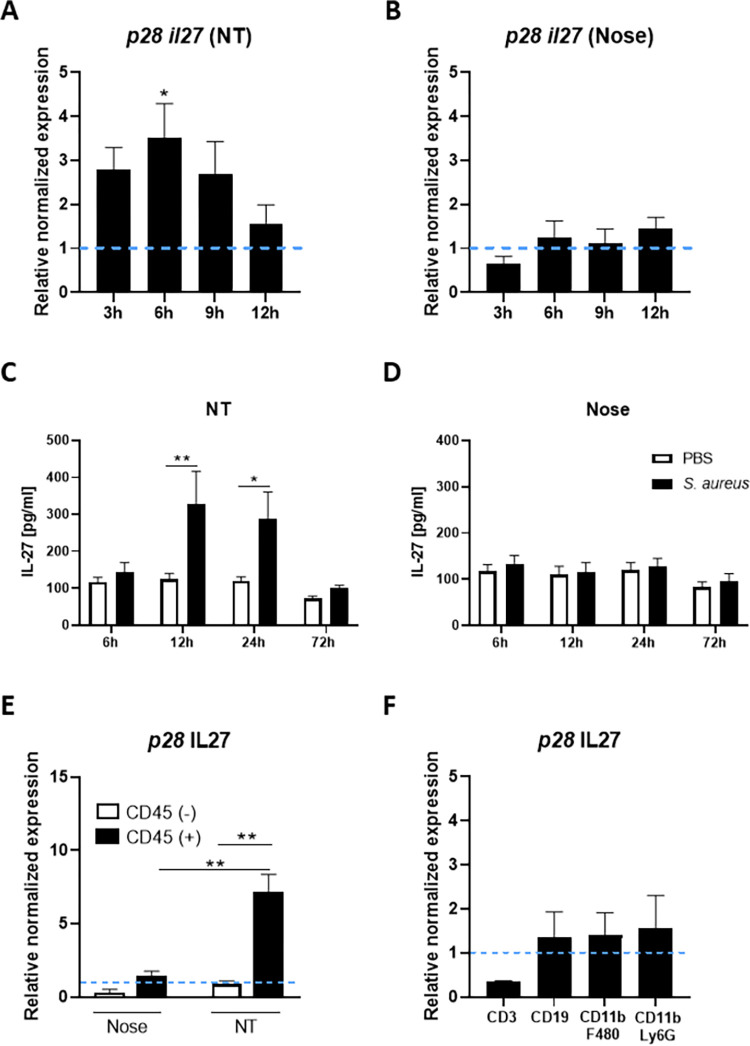
Fig 4. IL-27 is rapidly upregulated in the nasal cavity during S. aureus colonisation.
WT mice were colonised with S. aureus Newman SmR (2 × 108 colony-forming units/nose) or administered with PBS only. At 3h, 6h, 9h and 12h post-colonisation mice were culled, tissue homogenized and RNA extracted from nose and nasopharyngeal tissue (NT) homogenates. IL-27 gene expression in the NT (A) and nose (B) was established using qRT-PCR. The messenger RNA values were expressed as mean relative expression ±s.e.m. and was compared with baseline IL-27 expression from PBS-treated mice after normalizing to 18S RNA expression (Experimental unit = 1 mouse, n = 5, per group, total # animals used 40, data generated from 2 independent experiments). At 6h, 12h, 24h and 72h noses and NT were homogenized in PBS and protein levels of IL-27 (C & D) were determined by ELISA. Values are expressed as mean protein concentration ±s.e.m. (Experimental unit = 1 mouse, n = 5–10, per group, total # animals used 70, data generated from 2 independent experiments). At 6h post-colonisation noses and NT were excised, tissue digested and CD45+ and CD45- cells isolated by MACs sorting prior to assessment of IL-27 mRNA expression (E) (Experimental unit = Tissue was pooled from n = 10 mice and the experiment was repeated twice, total # of animals used 20). The CD45+ fraction was also sorted into CD3+, CD19+, CD11b+ F480+ and CD11b+ Ly6G+ F480- using flow cytometry cell sorting (F). IL-27 mRNA expression was determined by RT-PCR for each fraction (Experimental unit = tissue was pooled from n = 15 mice and the experiment was repeated twice, total number of animals used 30). The same mice were used as in Fig 1. Statistical analysis was performed using two-way analysis of variance or student t test. *P≤0.05; **P≤0.01,*** P≤0.00.