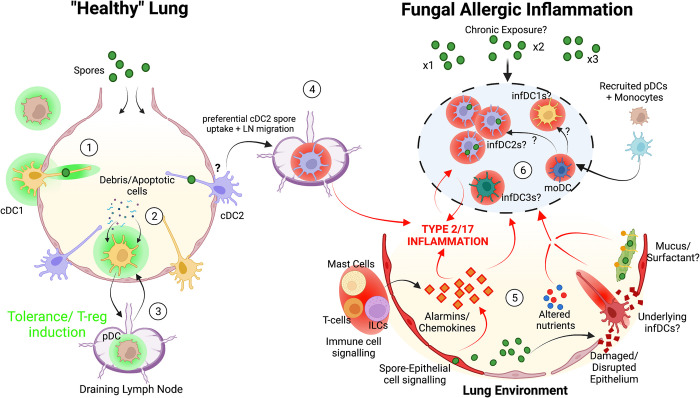
Fig 2. Understanding how DC induction of fungal allergic inflammation is shaped by the lung environment.
In health (left), DCs predominantly reside in the tissue but can project dendrites into the airway to sample antigen. (1) As AlvMΦs predominantly clear inhaled spores [43], exposure of DCs to fungal antigen is minimal reducing potential for inflammatory responses. (2) DC subsets, especially cDC1s, assume housekeeping duties (e.g., clearance of apoptotic cells) maintaining a tolerogenic phenotype. (3) Upon migration to draining LN lung DCs, in concert with other subsets such as pDCs, induce T-reg generation further maintaining an immuno-regulatory lung environment. (4) Fungal allergic inflammation is initiated upon cDC2 acquisition of Af spores and migration to the draining LN where they can prime adaptive CD4+ T cell responses (right). (5) While the precise mechanisms by which cDC2 mediate these responses to spores is unclear, the lung environment is known to directly influence this process. Fungal secretory products (including proteases) in the airway lumen can not only activate DCs directly, but also damage the epithelial barrier. This allows spores to move beyond the epithelial barrier and potentially activate cDC2s in the deeper underlying tissue. Furthermore, epithelial cell responses to fungi and/or barrier damage triggers the release of alarmins, chemokines, cytokines, and DAMPs (e.g., CCL2, IL-6, IL-33, and TSLP173, [174,175]), which can further activate DCs to promote allergic response. In addition, ILCs and mast cells (which can be activated by epithelial signals) further promote type 2 and type 17 cytokine which further conditions DCs to exacerbate allergic inflammation [68,145,180,184]. Other lung environmental factors such as altered nutrient availability and increased surfactant/ mucus concentrations [45,158] can further shape DC responses. (6) These features can lead to the formation of several inflammatory DC states (infDC1, infDC2, and infDC3) and possibly DCs differentiated from monocytes (moDCs) which further amplify and sustain fungal allergic inflammatory disease. Figures were created with BioRender.com. AlvMΦ, alveolar macrophage; CCL, chemokine ligand; DC, dendritic cell; IL, interleukin; moDC, monocyte-derived dendritic cell TSLP, thymic stromal lymphopoietin.