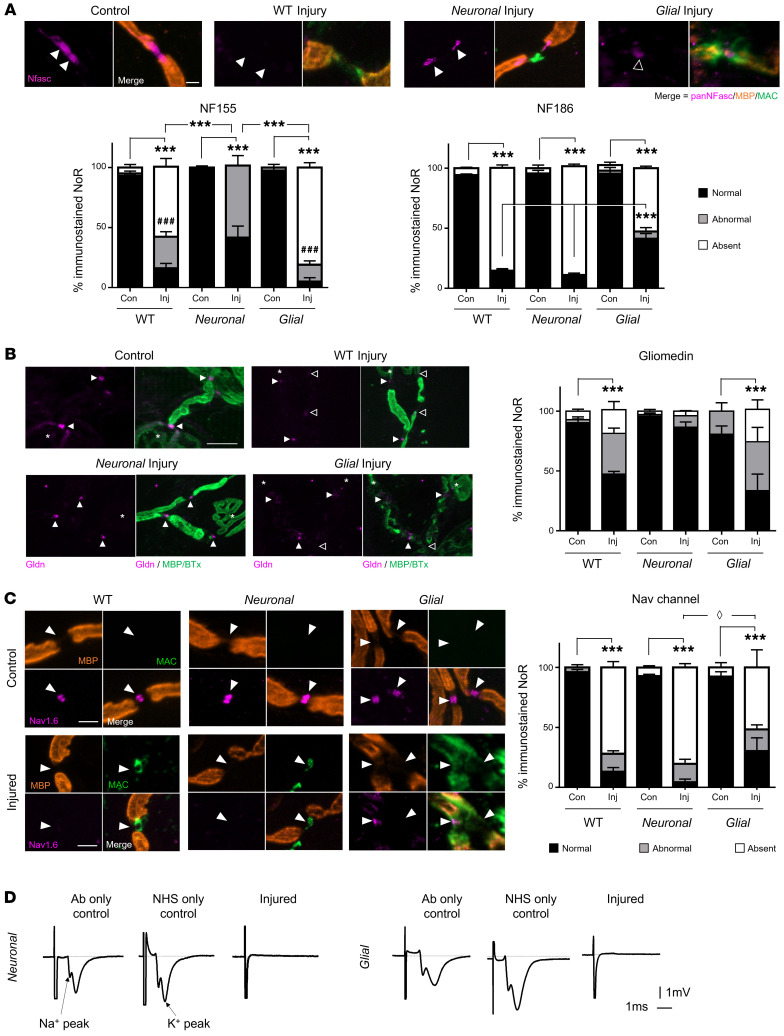
Figure 3. Differential disruption to the node of Ranvier when neuronal and glial membranes are injured selectively ex vivo.
Triangularis sterni nerve–muscle preparations from WT, Neuronal, and Glial mice were treated ex vivo with anti-GM1 Ab and a source of complement (injury, Inj) or anti-GM1 Ab alone (control, Con). Disruption to nodal protein (magenta) organization at the node of Ranvier (NoR) due to injury was assessed; the site of expected staining is indicated by arrowheads for each marker. Representative images demonstrate normal nodal protein localization in all control tissue and absent or abnormal staining in injury groups, which coincides with nodal complement deposition (A and C, green). (A) A pan-neurofascin (Nfasc) Ab was used to assess paranodal NF155 (closed arrowheads) and nodal NF186 (open arrowhead). (B) SC microvilli marker gliomedin (Gldn) immunostaining at NoRs was assessed compared to controls. Asterisks indicate motor nerve terminals. (C) Changes to normal (black bars) Nav1.6 labeling were observed in injured tissue from all genotypes compared with associated controls. Diamond defines statistical comparisons of absent immunostaining (white bars). (D) Perineural recordings from distal motor nerves were performed on tissue from Neuronal and Glial mice treated with anti-GM1 Ab only, a source of complement (normal human serum, NHS) only, or a combination of Ab and NHS (injured). Representative recordings from 1 mouse per treatment demonstrate that normal Na+ and K+ waveforms were lost when the tissue was injured. Scale bar: 5 μm. Results are represented as the mean ± SEM. n = 3/genotype/treatment: 13–36 NoRs/mouse (median = 24, pNFasc); 15–33 NoRs/mouse (median = 19, gliomedin); and 11–30 NoRs/mouse (median = 23, Nav1.6) were analyzed. *P < 0.05, **P < 0.01, ***P < 0.001 (for comparisons between normal immunostaining); ###P < 0.001 (for abnormal NF155 immunostaining in Neuronal injury group compared to WT or Glial imjury in A) compared with control by 2-way ANOVA with Tukey’s post hoc test.