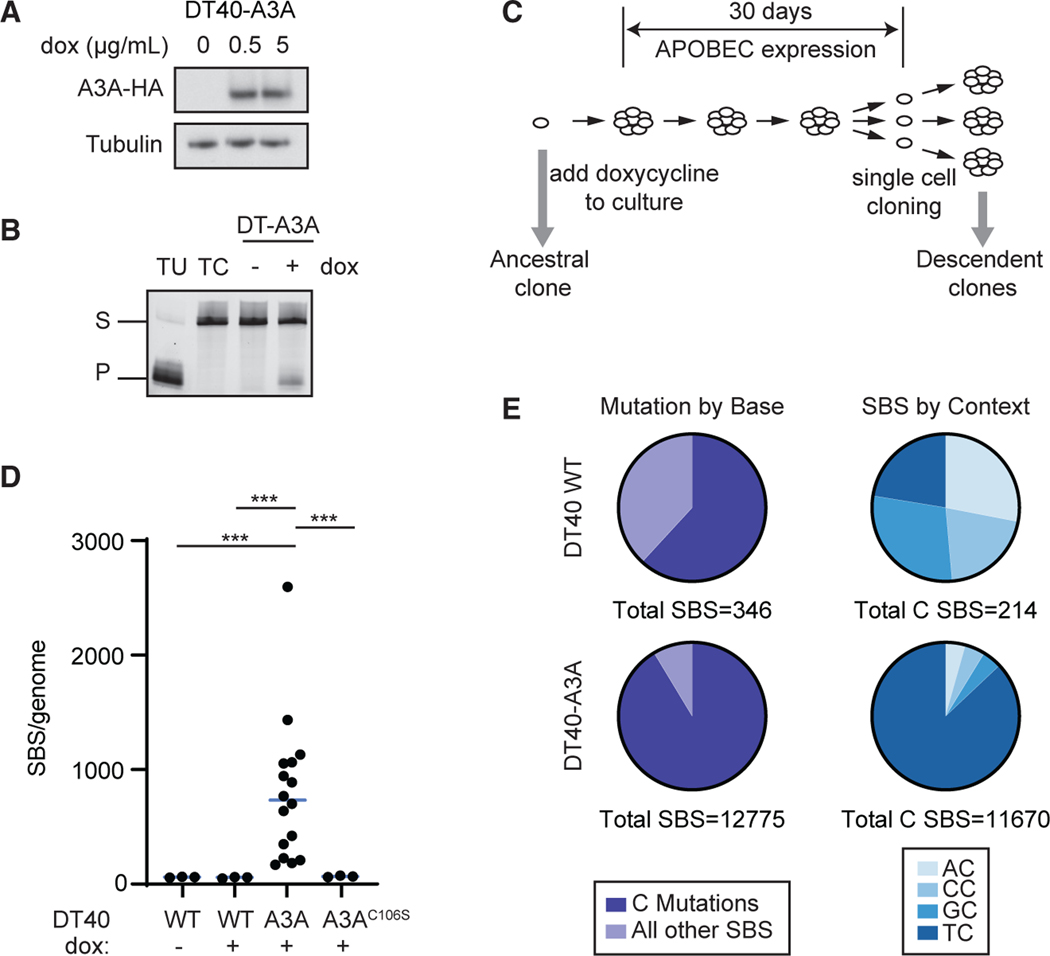
Figure 1. The genome-wide spectrum of mutations generated by human APOBEC3A in DT40 cells.
(A) Human APOBEC3A (A3A) was expressed inavian DT40 cells. Doxycycline (dox)-induced A3A expression was evaluated by immunoblot. A3A is detected by a hemagglutinin (HA) tag. The image is representative of two biological replicates.
(B) Deaminase activity in DT40-A3A cells. Lysates were incubated with a ssDNA oligonucleotide containing a single cytosine. Cytosine deamination followed by addition of uracil-DNA glycosylase (UDG) results in an abasic site; incubation with NaOH results in oligonucleotide cleavage. Substrate (S) and product (P) bands are visualized by gel electrophoresis. Oligonucleotides that contain a single uracil or cytosine (TU and TC), incubated in the absence of cell lysate, are controls. The image is representative of three biological replicates.
(C) Experimental schematic for evaluating A3A mutagenesis. A3A expression was induced in a DT40-A3A ancestral clone for 30 days. Subsequent single cell selection yielded descendant clones (n = 16), which were evaluated by whole-genome sequencing (WGS). In parallel, three control populations were cultured, sequenced, and analyzed: DT40 wild type (WT) untreated (n = 3), WT dox treated (n = 3), and DT40-A3AC106S (catalytically inactive A3A mutant, n = 3).
(D) Number of SBS mutations per genome. Each dot represents the base substitution burden within an individual descendant clone. Statistical analysis was performed by two-tailed t test; the bar indicates the median. ***p < 0.001.
(E) Total cytidine mutations. Left: cumulative cytidine mutations shown as a percentage of all SBSs in descendant clone genomes from WT and DT40-A3A cells. Right: dinucleotide contexts in which C base substitutions are shown as fractions of all mutated cytidines.