Abstract
NAD+—dependent histone deacetylases (sirtuins 1–7) have been shown to be involved in various pathophysiological conditions including their involvement in cardiovascular, cancerous, neurodegenerative, immune dysregulation and inflammatory conditions. This study investigates the inflammomodulatory potential of resveratrol (RES), a sirtuin activator and sirtinol (SIR), a sirtuin inhibitor in lipopolysaccharide (LPS)-induced model of sickness behaviour in mice. Male Swiss albino mice were divided into five groups (n = 6) consisting of saline (SAL), LPS, RES, SIR, and fluoxetine (FLU) respectively, each group except LPS was prepared by intraperitoneally (i.p.) administration of SAL (10 mL/kg), RES (50 mg/kg), SIR (2 mg/kg) and FLU (10 mg/kg). Thirty minutes after the treatments, all the groups, except SAL were administered LPS (2 mg/kg, i.p.). The behavioural assays including, open field test, forced swim test, and tail suspension tests were conducted 1 h after LPS challenge. LPS administration significantly reduced the locomotor activity along with inducing a state of high immobility and that was prevented by pretreatment with RES and SIR. Further, various proinflammatory cytokines (TNF-α, IL-6, and IL-1β), and oxidative stress markers (MDA and GSH) were found to be significantly elevated in the brain homogenates after LPS treatment. SIR pretreatment abrogated the LPS-induced neuroinflammatory and oxidative stress changes, whereas RES was only effective in reducing the oxidative stress and TNF-α levels. The results of this study speculate that the role of SIRT modulators in neuroinflammatory conditions could vary with their dose, regimen and chemical properties. Further studies with detailed molecular and pharmacokinetic profiling will be needed to explore their therapeutic potentials.
Keywords: Sickness behaviour, SIRT, Resveratrol, Sirtinol, Neuroinflammation, Cytokines
Introduction
Neuroinflammation is one major underlying cause of several CNS-related diseases (DiSabato et al. 2016). Lipopolysaccharide (LPS), an endotoxin that acts as a Pathogen-Associated Molecular Pattern (PAMPs) and binds to the Toll-like Receptors (TLRs) and triggers neuroinflammatory response. In animal models of neuroinflammation, LPS is administered peripherally, and it leads to an acute sickness behaviour followed by depressive-like state in a biphasic manner (Basu Mallik et al. 2016; 2021; Moraes et al. 2017). Acute sickness triggered by proinflammatory cytokines (IL-1, IL-6 and TNF-α) in response to PAMPs, is therefore an organized strategy to counteract infecting pathogens (Dantzer 2009). Thus, a phenomenological overlap is seen between sickness behaviour and early stages of clinical depression due to the circulatory cytokines (Maes et al. 2012).
Amongst the mammalian histone deacetylases (HDACs), HDAC1-11 are classified as classical zinc-dependent, and HDAC1-7 as nicotinamide adenine dinucleotide (NAD+)-dependent sirtuins (SIRT) (Lugrin et al. 2013). HDACs are further sub-grouped into various classes, and they catalyse the cleavage of acetyl groups from lysine residues (Lugrin et al. 2013). Deacetylation of histones causes gene repression, and modulates various non-histone proteins (PPARγ, PGC-1α, p53, NF-κB, p38 MAPK, FOXO1 and FOXO3). It also affects various biological and pathological processes involved in neurodegenerative, auto-immune, cardiovascular and oncologic conditions (Shakespear et al. 2011; Lugrin et al. 2013; Paraíso et al. 2013; Jęśko et al. 2017). Along with the known clinical anticancer properties, classical HDAC inhibitors have also been shown to possess anti-inflammatory and immunomodulatory activities (Shakespear et al. 2011; Jenke et al. 2021).
In vitro data suggests that SIRT1 activation by resveratrol (RES) reduces neuroinflammation by decreasing the levels of IL-1β, IL-6, matrix metalloprotein-9 and iNOS along with decreased acetylated p53 and cleaved caspase 3 (Zhang et al. 2020). Furthermore, chronic RES treatment reversed chronic unpredictable mild stress (CUMS)-induced protein changes leading to increased expression of SIRT1, p-CREB, CREB, and BDNF while reduced miR-134 levels (Shen et al. 2018). Moreover, being a polyphenolic compound RES has been well documented to reduce oxidative and nitrosative stress markers ((Palsamy and Subramanian 2011; Jing et al. 2013; Gordish and Beierwaltes 2014; Park and Pezzuto 2015).
On the other hand, silencing of SIRT2 in microglia reduced the LPS-induced microglial activation thereby lowering the TNF-α and IL-6 levels (Chen et al. 2015). Similarly, LPS-induced ROS generation and NF-κB activation was shown to be significantly reduced in SIRT2 knockout mice (Lee et al. 2014). Moreover, the CONVERGE consortium (CONVERGE consortium 2015) has linked the SIRT1 gene with major depressive disorder. Further studies substantiate that after chronic social defeat stress, SIRT1 expression increases. and administration of RES directly into the nucleus accumbens also activates SIRT1 and produces a phenotype with increased anxiety and depression-like behaviour (Kim et al. 2016).
Interestingly, both sirtinol (SIR), and RES are polyphenolic in structure and can act on multiple cellular targets including steroid-hormone mediated pathways and xenobiotic metabolisms (Wang et al. 2013). The polyphenolic structural similarities between these two compounds could be responsible for an overlap in their biological activities. Based on this background we chose to compare the effects of both RES and SIR on LPS-induced psychopharmacological parameters and brain cytokines which are involved in neuroinflammatory conditions.
Materials and methods
Animals
Male Swiss albino mice, (8–10 weeks old, 20–30 g) were used in this study and were procured from the inbred strains of Central Animal research Facility (CARF), Manipal Academy of Higher Education (MAHE), Manipal for the study. All the experimental procedures were approved by the Institutional Animal Ethics Committee (IAEC) of Manipal Academy of Higher Education (IAEC/KMC/25/2020 dated 22/02/2020) and were performed in accordance with the guidelines set out in compliance with the National Institutes of Health Guide for Care and Use of Laboratory Animals (Publication No. 85–23, revised 1985). Animals were housed in groups of 6 under controlled laboratory conditions, maintained at 12 h day and night cycle with free access to food and water.
Chemicals and Reagents
Lipopolysaccharide (LPS) (Escherichia coli serotype O111:B4), fluoxetine hydrochloride, 2-thiobarbituric acid (TBA), sodium dihydrogen phosphate anhydrous, disodium hydrogen phosphate anhydrous and trichloroacetic acid were purchased from Sigma-Aldrich (Sigma-Aldrich Co. LLC (St Louis, MO, USA). Resveratrol and sirtinol were procured from Abcam (Abcam plc, Cambridge, UK). All other chemicals used in this study were of analytical grade.
Drug treatments
Animals were randomised based on the body weights and we allocated into five groups (n = 6). Group 1 served as control (SAL); group 2 as LPS (SAL + LPS); group 3 as resveratrol treatment (RES + LPS), group 4 as sirtinol treatment (SIR + LPS), and group 5 as fluoxetine treatment (FLU + LPS). All the treatments were administered by intraperitoneal (i.p.) route. SAL and LPS groups were administered normal saline at a dose of 10 mL/kg. RES, SIR and FLU groups were treated with RES (50 mg/kg), SIR (2 mg/kg) and FLU (10 mg/kg) respectively. All animals (except SAL group) received a single injection of LPS (2 mg/kg) 30 min after the treatment. Behavioural assays were performed within 1–2 h of LPS administration and were video recorded. Animals were euthanised at 3 h post LPS injections and brain samples were isolated and stored at -80 °C till further analysis. Tissue samples were homogenised using chilled phosphate buffer (0.1 M, pH 7.4) for antioxidant and cytokine level estimations.
Behavioural assays
A series of behavioural assays were performed, including open field test (OFT) to measure the spontaneous activity, forced swimming test (FST) and tail suspension test (TST), for the measurement of immobility state. All the assays followed the procedures as described earlier (Wang et al. 2013; Basu Mallik et al. 2016; Mudgal et al. 2019). OFT was measured as the number of line crossings and rearing in a plexiglass chamber (30 cm × 30 cm × 90 cm), where the chamber was divided into 9 equal virtual quadrants of 10 cm × 10 cm. FST was assessed by calculating the total time spent by the animal in an immobile state over the 5 min of observational period in a transparent plexiglass cylindrical tank (30 × 20 cm), whereas TST was recorded as the immobility time with the animals individually hung for a period of 5 min at 15 cm away from the nearest surface.
Estimation of brain cytokine and lipid peroxidation levels
Cytokines, namely, IL-6, TNF-α and IL-1β were estimated using commercially available kits (Invitrogen, California, USA). Lipid peroxidation (LPO) and reduced glutathione (GSH) were performed as detailed in earlier (Sahu et al. 2019; Mudgal et al. 2019). Brain homogenates were incubated with equal volumes of TBA at 90 °C for 10 min. Malondialdehyde (MDA) formed was measured spectrophotometrically at 532 nm. Similarly, GSH was estimated by the absorbance of GSH and DTNB complex at 412 nM. Total protein estimation was carried out using Pierce™ BCA Protein Assay Kit (ThermoFisher Scientific, USA), as per the manufacturer’s instructions.
Statistical analysis
All data sets were analysed for statistical significance utilising GraphPad Prism 9.0.0 (Graph Pad Software Inc., San Diego, CA, USA). Values are expressed as means ± S.E.M. Experimental groups were compared against the control groups using one-way analysis of variance (ANOVA) followed by Dunnett’s multiple comparison test. A “p” value of < 0.05 was considered to be statistically significant.
Results
Effect of RES and SIR on behavioural parameters
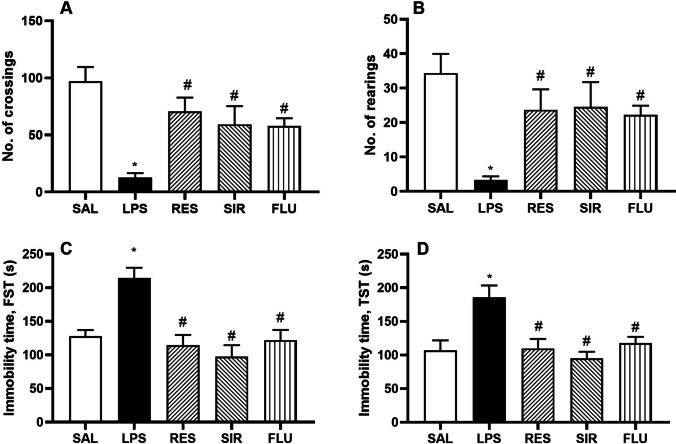
Administration of LPS produced a significant reduction in the locomotor activity (LMA) as assessed by the number of crossings (12.67 ± 3.89 vs 97.00 ± 12.53 of SAL treated group; Fig. 1A), and number of rearing (3.33 ± 1.02 vs 34.33 ± 5.59 of SAL treated group; Fig. 1B). Pretreatment of the animals with RES, SIR and FLU (70.50 ± 12.30; 59.33 ± 15.94 and 57.83 ± 6.83 respectively, F [4, 25] = 7.46, p < 0.05, Fig. 1A) significantly reduced the LPS-induced effect on LMA and rearing (23.67 ± 5.98; 24.50 ± 7.22; 22.17 ± 2.75 respectively, F [4, 25] = 4.98, p < 0.05, Fig. 1B).
Fig. 1.
Effect of saline (SAL), resveratrol (RES; 50 mg/kg), sirtinol (SIR; 2 mg/kg) and fluoxetine (FLU; 10 mg/kg) on LPS-induced behavioural changes. Number of crossings (1A); number of rearing (1B); immobility time (s) (FST) (1C); immobility time (s) (TST) (1D). *p < 0.05 as compared to SAL group; #p < 0.05 as compared with LPS group
In FST, LPS administration led to a significantly increased immobile state in all the animals (214.30 ± 15.42 s, vs 128.00 ± 9.02 s of SAL treated group) (Fig. 1C). Interestingly, all the pretreatments, including RES, SIR and FLU (114.30 ± 15.40; 97.67 ± 16.90; 122.00 ± 15.11 respectively, F [4, 25] = 9.73, p < 0.05) significantly improved this LPS-induced increase in the immobility time.
Similarly, the immobility time in tail suspension test (TST) was also significantly increased by LPS treatment (185.70 ± 17.68 s) as compared to SAL treated group (106.80 ± 14.94 s) (Fig. 1D). Pretreatment of the animals with RES, SIR and FLU was found to produce significant protection against the impact of LPS on TST immobility time (109.70 ± 14.13 s, 94.83 ± 10.12 s and 117.50 ± 9.49 s respectively, F [4, 25] = 6.99, p < 0.05).
Effect of RES and SIR on oxidative stress markers
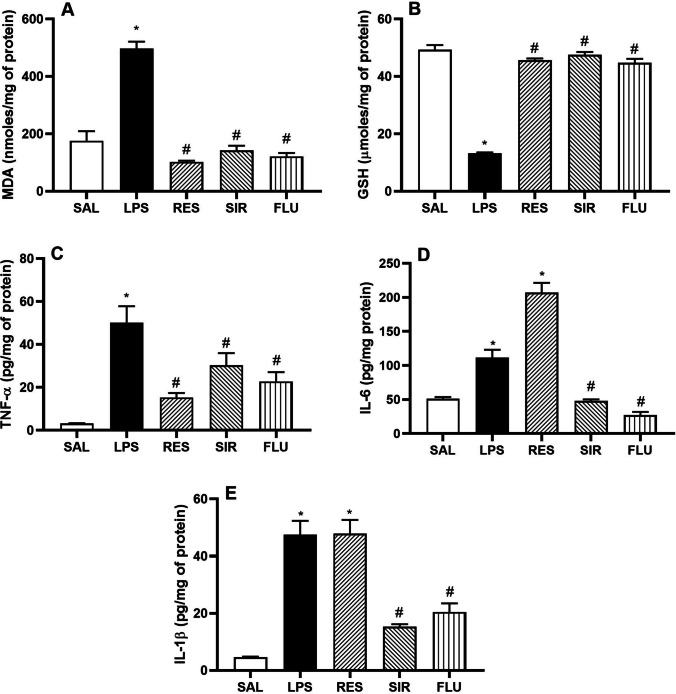
Oxidative stress markers of MDA and GSH were quantified in the brain tissue homogenates of all treatment groups. LPS administration caused a significant increase in lipid peroxidation as quantified by MDA levels (nmol/mg of protein) (496.70 ± 24.38 vs 175.80 ± 33.32 of SAL treated group; Fig. 2A), and it also led to a considerable decrease in total GSH levels (μmol/mg of protein) (13.27 ± 0.26 vs 49.25 ± 1.65 of SAL treated group; Fig. 2B). Pretreatment of the animals with RES (102.20 ± 4.06), SIR (142.20 ± 16.34), and FLU (121.20 ± 11.89, F = [4, 25] = 63.07, p < 0.05, Fig. 2A) offered significant protection against LPS-induced lipid peroxidation. Similarly, the GSH levels were also preserved by pretreatment with RES (45.64 ± 0.62), SIR (47.55 ± 0.99), and FLU (44.80 ± 1.30, F [4, 25] = 194.10, p < 0.05, Fig. 2B).
Fig. 2.
Effect of saline (SAL), resveratrol (RES; 50 mg/kg), sirtinol (SIR; 2 mg/kg) and fluoxetine (FLU; 10 mg/kg) on LPS-induced changes in brain homogenates. MDA levels (nmoles/mg protein) (2A); GSH (µmoles/mg protein) (2B); TNF-α (pg/mg protein) (2C), IL-6 (pg/mg protein) (2D), and IL-1β (pg/mg protein) (2E). *p < 0.05 as compared to SAL group; #p < 0.05 as compared with LPS group
Effect of RES and SIR on brain inflammatory markers
Acute administration of LPS significantly increased the levels of proinflammatory cytokines including, TNF-α (50.23 ± 7.60 vs 3.14 ± 0.18 pg/mg protein of SAL), IL-6 (111.50 ± 11.47 vs 51.23 ± 2.49 pg/mg protein of SAL), and IL-1β (47.46 ± 4.93 vs 4.62 ± 0.23 pg/mg protein of SAL, Fig. 2A and 2B). Pretreatment of the animals with RES (15.27 ± 2.08 pg/mg protein) significantly reduced the TNF-α levels, however, both IL-6 (207.20 ± 14.07 pg/mg protein) and IL-1β (47.86 ± 4.81 pg/mg protein) stayed significantly elevated with RES pretreatment. On the other hand, pretreatment with SIR and FLU significantly reduced TNF-α (30.34 ± 5.61 and 22.78 ± 4.35 pg/mg protein respectively, F [4, 20] = 18.60, p < 0.05, Fig. 2C), IL-6 (48.02 ± 2.02 and 27.21 ± 4.51 pg/mg protein respectively, F [4, 21] = 103.8, p < 0.05, Fig. 2D), and IL-1β (15.26 ± 0.94 and 20.48 ± 2.99 pg/mg protein respectively, F [4, 20] = 46.68, p < 0.05, Fig. 2E).
Discussion
Peripheral administration of bacterial endotoxin (LPS), in animals leads to a biphasic response in both behavioural and biochemical parameters. The acute phase of “sickness behaviour” peaks within 3–4 h of LPS administration and is expressed by a group of symptoms, including anhedonia, slowness in initiation of movement, decreased mobility, exploration and grooming, hunched posture, and hyperalgesia (Painsipp et al. 2011; Berk et al. 2013). In our study, a single administration of LPS in animals led to a significant reduction in both horizontal and vertical activities, as indicated by the significantly reduced number of line crossings and rearing in the open field arena. Furthermore, the immobile state was considerably increased in both FST and TST. Both RES and SIR significantly improved the spontaneous locomotion in LPS treated animals, as the number of line crossings and rearing were significantly increased. Moreover, both pretreatments significantly reduced the immobility time in both FST and TST. These behavioural changes correlated with the neuronal pro-inflammatory cytokines (TNF-α, IL-6 and IL-1β), and oxidative stress markers (MDA and GSH) changes.
LPS acts as a ligand for toll-like receptors (TLR 2 and 4), and it causes the translocation of nuclear factor (NF-κB) by dissociation of inhibitory protein κB (IκB). This initiates a cascade of events leading to activation of immune and inflammatory systems, including expression of cytokines and chemokines, cell proliferation and migration (Yang et al. 1998; Kim et al. 2012; Salt and Palmer 2012; Búfalo et al. 2013). Intraperitoneal injection of LPS compromises the integrity of the blood brain barrier (BBB) by interfering with the physical barriers through damage to endothelial junctions and glycocalyx damage (Wiesinger et al. 2013; Varatharaj and Galea 2017). In our study, LPS produced a significant increase of tested brain cytokines, namely TNF-α, IL-6 and IL-1β. These results correspond with our earlier studies (Mudgal et al. 2019, 2020) where we have shown that the levels of both TNF-α and IL-6 peak rapidly in the plasma and producing a neuroinflammatory state in the brain.
RES has been shown to activate SIRT1 and thereby reducing NF-κB activation by deacetylating p65 subunit (Moon et al. 2013; Jiao and Gong 2020). The most interesting finding of our study was that at the employed doses and schedule, RES reduced the oxidative stress markers and TNF-α in a potent manner, however, there was no noticeable effect of RES on LPS-induced increase in IL-6 and IL-1β. In coherence with these results, it has been shown that in LPS-activated peripheral blood leucocytes, RES produces an overall inhibitory effect on cytokines and chemokines production only during un-stimulated conditions. Where, these pro-inflammatory mediators are secreted in low concentrations as compared to those produced by LPS-stimulated conditions (Richard et al. 2005; Schwager et al. 2017). Further to this, more evidence supports that the production of IL-6 and IL-1β is enhanced by RES in a concentration dependent manner (Schwager et al. 2017). Ex vivo RES treated peripheral blood mononuclear cells from osteoarthritis patients produced higher IL-6 levels in a dose-dependent manner. These results indicate that IL-6 expression might be particularly controlled by SIRT1 (Wendling et al. 2013). Similarly, activation of SIRT1 causes elevation of IL-6 and TNF-α and marginally altering the immune responses in vitro, however, the impact of SIRT1 inhibition or activation on the function of other immune cells remains unclear (Mourits et al. 2021). It is noteworthy that this study involved an acute dosing of RES. Furthermore, IL-1β and IL-6 contribute to Th-lymphocyte differentiation and function, and high levels of these cytokines would prime for adaptive immune response (Mauer et al. 2014). Therefore, chronic treatment with RES is essential before any conclusive statements can be delivered.
Another important finding of this study was that SIR, a nonselective SIRT inhibitor was able to ameliorate LPS-induced upregulation of pro-inflammatory cytokines. Our findings are not exactly in coherence with some of the existing studies where SIRT1 inhibition is involved as a contributing factor for various pathological conditions (Orecchia et al. 2011). We here propose that the effect of SIRT inhibition is dose and regimen dependent, where low and acute dosing of SIR reduces the neuroinflammatory impact of LPS administration. Interestingly, Lugrin et al. (2013) have shown that both SIR and its structural analogue, cambinol impaired the production of IL-6, and TNF-α from macrophages stimulated with LPS. It was suggested that selective SIRT1 and/or SIRT 2 inhibition alone was not effective in reducing the production of these pro-inflammatory cytokines, and SIR could be exhibiting these properties by targeting more than just SIRT1 and SIRT2 (Lugrin et al. 2013). Furthermore, acute pretreatment with SIR at the comparable doses (2.5 and 5 mg/kg, i.p.) has shown to significantly reduce neutrophil elastase induced paw edema and LPS-induced acute lung injury (Tsai et al. 2015). SIR also possess anti-inflammatory properties by affecting the chemokine and adhesion molecule expression (Orecchia et al. 2011) and produces antiproliferative effects in NF-κB p65-independent manner (Fong et al. 2014).
We included FLU as a standard selective serotonin reuptake inhibitor (SSRI) for this study. Apart from its clinically established antidepressant activity FLU has been shown to be effective against LPS-induced inflammation and microglial activation. Moreover, it also reduces inflammatory markers, and oxidative stress along with improvement in the behavioural parameters of FST and TST (Ghosh et al. 2020). Our results are coherent with the existing reports, as FLU significantly reduced the LPS-induced neuroinflammatory and oxidative stress markers, along with normalising the behavioural changes.
This study compared the effects of both SIRT inhibitor (SIR) and activator (RES) is an acute neuroinflammation model of LPS-induced sickness behaviour. We found that the acute effects of SIRT inhibition were more pronounced in reducing the inflammation-induced sickness behaviour in mice. We have substantiated our findings with the existing literature reports. However, it is noteworthy that these SIRT modulators act by various pleiotropic mechanisms, and the effects could further be dependent on the dose, route, schedule of administration and the chemical nature of molecules. Further investigations utilising chronic dosing regimens of these compounds along with their pharmacokinetic profiling would be required to supplement these findings. Moreover, additional studies utilising models of neuroinflammation with multiple injections of endotoxins would be more specific to understand this neuroinflammation-induced neurodegenerative changes.
Acknowledgements
“CSIR-Direct SRF Fellowship” to MK and “TMA Pai PhD Scholarship to NR” are truly acknowledged. The authors also thank Dr KSR Pai, Head of Pharmacology Department, Manipal College of Pharmaceutical Sciences, Manipal Academy of Higher Education (MAHE), Manipal, for providing facilities to carry out this project, and Mr Sridhara Prabhu, CARF, MAHE, Manipal for facilitating the animal experiments and providing necessary support to carry out this project.
Author contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by [Manas Kinra] [Niraja Ranadive] [Jayesh Mudgal] [Yuqing Zhang] [ Anusha Govindula] and [Devinder Arora]. The first draft of the manuscript was written by [Manas Kinra] [Jayesh Mudgal] [Devinder Arora] [Yuqing Zhang] [Madhavan Nampoothiri], and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions This study was financially supported by Intramural Funding (MCOPS/IMF/2019), MAHE, Manipal to JM and School of Pharmacy & Pharmacology research funds to DA, Griffith University.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request
Declarations
Conflict of interest
The authors declare no conflict of interest associated with this publication.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Manas Kinra, Niraja Ranadive, Jayesh Mudgal and Yuqing Zhang contributed equally to this work.
References
- Basu Mallik S, Mudgal J, Hall S, Kinra M, Grant GD, Nampoothiri M, Anoopkumar-Dukie S, Arora D (2021) Remedial effects of caffeine against depressive-like behaviour in mice by modulation of neuroinflammation and BDNF. Nutr Neurosci 1-9. 10.1080/1028415X.2021.1906393 [DOI] [PubMed]
- Basu Mallik S, Mudgal J, Nampoothiri M, Hall S, Dukie SA, Grant G, Rao CM, Arora D. Caffeic acid attenuates lipopolysaccharide-induced sickness behaviour and neuroinflammation in mice. Neurosci Lett. 2016;632:218–223. doi: 10.1016/j.neulet.2016.08.044. [DOI] [PubMed] [Google Scholar]
- Berk M, Williams LJ, Jacka FN, O'Neil A, Pasco JA, Moylan S, Allen NB, Stuart AL, Hayley AC, Byrne ML, Maes M. So depression is an inflammatory disease, but where does the inflammation come from? BMC Med. 2013;11:200. doi: 10.1186/1741-7015-11-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Búfalo MC, Ferreira I, Costa G, Francisco V, Liberal J, Cruz MT, Lopes MC, Batista MT, Sforcin JM. Propolis and its constituent caffeic acid suppress LPS-stimulated pro-inflammatory response by blocking NF-κB and MAPK activation in macrophages. J Ethnopharmacol. 2013;149:84–92. doi: 10.1016/j.jep.2013.06.004. [DOI] [PubMed] [Google Scholar]
- Chen H, Wu D, Ding X, Ying W. SIRT2 is required for lipopolysaccharide-induced activation of BV2 microglia. NeuroReport. 2015;26:88–93. doi: 10.1097/WNR.0000000000000305. [DOI] [PubMed] [Google Scholar]
- CONVERGE consortium Sparse whole-genome sequencing identifies two loci for major depressive disorder. Nature. 2015;523:588–591. doi: 10.1038/nature14659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R. Cytokine, sickness behavior, and depression. Immunol Allergy Clin North Am. 2009;29:247–264. doi: 10.1016/j.iac.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiSabato DJ, Quan N, Godbout JP. Neuroinflammation: the devil is in the details. J Neurochem. 2016;139(Suppl 2):136–153. doi: 10.1111/jnc.13607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong Y, Lin YC, Wu CY, Wang HM, Lin LL, Chou HL, Teng YN, Yuan SS, Chiu CC. The antiproliferative and apoptotic effects of sirtinol, a sirtuin inhibitor on human lung cancer cells by modulating Akt/β-catenin-Foxo3a axis. ScientificWorldJournal. 2014;2014:937051. doi: 10.1155/2014/937051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S, Choudhury S, Chowdhury O, Mukherjee S, Das A, Sain A, Gupta P, Adhikary A, Chattopadhyay S. Inflammation-induced behavioral changes is driven by alterations in Nrf2-dependent apoptosis and autophagy in mouse hippocampus: Role of fluoxetine. Cell Signal. 2020;68:109521. doi: 10.1016/j.cellsig.2019.109521. [DOI] [PubMed] [Google Scholar]
- Gordish KL, Beierwaltes WH. Resveratrol induces acute endothelium-dependent renal vasodilation mediated through nitric oxide and reactive oxygen species scavenging. Am J Physiol Renal Physiol. 2014;306:F542–F550. doi: 10.1152/ajprenal.00437.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenke R, Reßing N, Hansen FK, Aigner A, Büch T. Anticancer Therapy with HDAC Inhibitors: Mechanism-Based Combination Strategies and Future Perspectives. Cancers (Basel) 2021;13:634. doi: 10.3390/cancers13040634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jęśko H, Wencel P, Strosznajder RP, Strosznajder JB. Sirtuins and Their Roles in Brain Aging and Neurodegenerative Disorders. Neurochem Res. 2017;42:876–890. doi: 10.1007/s11064-016-2110-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao F, Gong Z (2020) The Beneficial Roles of SIRT1 in Neuroinflammation-Related Diseases. Oxid Med Cell Longev :6782872. 10.1155/2020/6782872. [DOI] [PMC free article] [PubMed]
- Jing YH, Chen KH, Kuo PC, Pao CC, Chen JK. Neurodegeneration in streptozotocin-induced diabetic rats is attenuated by treatment with resveratrol. Neuroendocrinology. 2013;98:116–127. doi: 10.1159/000350435. [DOI] [PubMed] [Google Scholar]
- Kim HD, Hesterman J, Call T, Magazu S, Keeley E, Armenta K, Kronman H, Neve RL, Nestler EJ, Ferguson D. SIRT1 Mediates Depression-Like Behaviors in the Nucleus Accumbens. J Neurosci. 2016;36:8441–8452. doi: 10.1523/JNEUROSCI.0212-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim IT, Ryu S, Shin JS, Choi JH, Park HJ, Lee KT. Euscaphic acid isolated from roots of Rosa rugosa inhibits LPS-induced inflammatory responses via TLR4-mediated NF-κB inactivation in RAW 264.7 macrophages. J Cell Biochem. 2012;113:1936–1946. doi: 10.1002/jcb.24062. [DOI] [PubMed] [Google Scholar]
- Lee AS, Jung YJ, Kim D, Nguyen-Thanh T, Kang KP, Lee S, Park SK, Kim W. SIRT2 ameliorates lipopolysaccharide-induced inflammation in macrophages. Biochem Biophys Res Commun. 2014;450:1363–1369. doi: 10.1016/j.bbrc.2014.06.135. [DOI] [PubMed] [Google Scholar]
- Lugrin J, Ciarlo E, Santos A, Grandmaison G, dos Santos I, Le Roy D, Roger T. The sirtuin inhibitor cambinol impairs MAPK signaling, inhibits inflammatory and innate immune responses and protects from septic shock. Biochim Biophys Acta. 2013;1833:1498–1510. doi: 10.1016/j.bbamcr.2013.03.004. [DOI] [PubMed] [Google Scholar]
- Maes M, Berk M, Goehler L, Song C, Anderson G, Gałecki P, Leonard B. Depression and sickness behavior are Janus-faced responses to shared inflammatory pathways. BMC Med. 2012;10:66. doi: 10.1186/1741-7015-10-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauer J, Chaurasia B, Goldau J, Vogt MC, Ruud J, Nguyen KD, Theurich S, Hausen AC, Schmitz J, Brönneke HS, Estevez E, Allen TL, Mesaros A, Partridge L, Febbraio MA, Chawla A, Wunderlich FT, Brüning JC. Signaling by IL-6 promotes alternative activation of macrophages to limit endotoxemia and obesity-associated resistance to insulin. Nat Immunol. 2014;15:423–430. doi: 10.1038/ni.2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon MH, Jeong JK, Lee YJ, Seol JW, Jackson CJ, Park SY. SIRT1, a class III histone deacetylase, regulates TNF-α-induced inflammation in human chondrocytes. Osteoarthritis Cartilage. 2013;21:470–480. doi: 10.1016/j.joca.2012.11.017. [DOI] [PubMed] [Google Scholar]
- Moraes MM, Galvão MC, Cabral D, Coelho CP, Queiroz-Hazarbassanov N, Martins MF, Bondan EF, Bernardi MM, Kirsten TB. Propentofylline Prevents Sickness Behavior and Depressive-Like Behavior Induced by Lipopolysaccharide in Rats via Neuroinflammatory Pathway. PLoS ONE. 2017;12:e0169446. doi: 10.1371/journal.pone.0169446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mourits VP, Helder LS, Matzaraki V, Koeken VACM, Groh L, de Bree LCJ, Moorlag SJCFM, van der Heijden CDCC, Keating ST, van Puffelen JH, Jaeger M, Joosten LAB, Netea MG. The role of sirtuin 1 on the induction of trained immunity. Cell Immunol. 2021;366:104393. doi: 10.1016/j.cellimm.2021.104393. [DOI] [PubMed] [Google Scholar]
- Mudgal J, Basu Mallik S, Nampoothiri M, Kinra M, Hall S, Grant GD, Anoopkumar-Dukie S, Davey AK, Rao CM, Arora D. Effect of coffee constituents, caffeine and caffeic acid on anxiety and lipopolysaccharide-induced sickness behavior in mice. J Funct Foods. 2020;64:103638. doi: 10.1016/j.jff.2019.103638. [DOI] [Google Scholar]
- Mudgal J, Nampoothiri M, Basu Mallik S, Kinra M, Hall S, Grant G, Anoopkumar-Dukie S, Rao CM, Arora D. Possible involvement of metformin in downregulation of neuroinflammation and associated behavioural changes in mice. Inflammopharmacology. 2019;27:941–948. doi: 10.1007/s10787-019-00638-w. [DOI] [PubMed] [Google Scholar]
- Orecchia A, Scarponi C, Di Felice F, Cesarini E, Avitabile S, Mai A, Mauro ML, Sirri V, Zambruno G, Albanesi C, Camilloni G, Failla CM. Sirtinol treatment reduces inflammation in human dermal microvascular endothelial cells. PLoS ONE. 2011;6:e24307. doi: 10.1371/journal.pone.0024307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Painsipp E, Köfer MJ, Sinner F, Holzer P. Prolonged depression-like behavior caused by immune challenge: influence of mouse strain and social environment. PLoS ONE. 2011;6:e20719. doi: 10.1371/journal.pone.0020719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palsamy P, Subramanian S. Resveratrol protects diabetic kidney by attenuating hyperglycemia-mediated oxidative stress and renal inflammatory cytokines via Nrf2-Keap1 signaling. Biochim Biophys Acta. 2011;1812:719–731. doi: 10.1016/j.bbadis.2011.03.008. [DOI] [PubMed] [Google Scholar]
- Paraíso AF, Mendes KL, Santos SH. Brain activation of SIRT1: role in neuropathology. Mol Neurobiol. 2013;48:681–689. doi: 10.1007/s12035-013-8459-x. [DOI] [PubMed] [Google Scholar]
- Park EJ, Pezzuto JM. The pharmacology of resveratrol in animals and humans. Biochim Biophys Acta. 2015;1852:1071–1113. doi: 10.1016/j.bbadis.2015.01.014. [DOI] [PubMed] [Google Scholar]
- Richard N, Porath D, Radspieler A, Schwager J. Effects of resveratrol, piceatannol, tri-acetoxystilbene, and genistein on the inflammatory response of human peripheral blood leukocytes. Mol Nutr Food Res. 2005;49:431–442. doi: 10.1002/mnfr.200400099. [DOI] [PubMed] [Google Scholar]
- Sahu P, Mudgal J, Arora D, Kinra M, Mallik SB, Rao CM, Pai KSR, Nampoothiri M. Cannabinoid receptor 2 activation mitigates lipopolysaccharide-induced neuroinflammation and sickness behavior in mice. Psychopharmacology. 2019;236:1829–1838. doi: 10.1007/s00213-019-5166-y. [DOI] [PubMed] [Google Scholar]
- Salt IP, Palmer TM. Exploiting the anti-inflammatory effects of AMP-activated protein kinase activation. Expert Opin Investig Drugs. 2012;21:1155–1167. doi: 10.1517/13543784.2012.696609. [DOI] [PubMed] [Google Scholar]
- Schwager J, Richard N, Widmer F, Raederstorff D. Resveratrol distinctively modulates the inflammatory profiles of immune and endothelial cells. BMC Complement Altern Med. 2017;17:309. doi: 10.1186/s12906-017-1823-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakespear MR, Halili MA, Irvine KM, Fairlie DP, Sweet MJ. Histone deacetylases as regulators of inflammation and immunity. Trends Immunol. 2011;32:335–343. doi: 10.1016/j.it.2011.04.001. [DOI] [PubMed] [Google Scholar]
- Shen J, Xu L, Qu C, Sun H, Zhang J. Resveratrol prevents cognitive deficits induced by chronic unpredictable mild stress: Sirt1/miR-134 signalling pathway regulates CREB/BDNF expression in hippocampus in vivo and in vitro. Behav Brain Res. 2018;349:1–7. doi: 10.1016/j.bbr.2018.04.050. [DOI] [PubMed] [Google Scholar]
- Tsai YF, Yu HP, Chang WY, Liu FC, Huang ZC, Hwang TL. Sirtinol inhibits neutrophil elastase activity and attenuates lipopolysaccharide-mediated acute lung injury in mice. Sci Rep. 2015;5:8347. doi: 10.1038/srep08347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varatharaj A, Galea I. The blood-brain barrier in systemic inflammation. Brain Behav Immun. 2017;60:1–12. doi: 10.1016/j.bbi.2016.03.010. [DOI] [PubMed] [Google Scholar]
- Wang TT, Schoene NW, Kim EK, Kim YS. Pleiotropic effects of the sirtuin inhibitor sirtinol involves concentration-dependent modulation of multiple nuclear receptor-mediated pathways in androgen-responsive prostate cancer cell LNCaP. Mol Carcinog. 2013;52:676–685. doi: 10.1002/mc.21906. [DOI] [PubMed] [Google Scholar]
- Wendling D, Abbas W, Godfrin-Valnet M, Guillot X, Khan KA, Cedoz JP, Baud L, Prati C, Herbein G. Resveratrol, a sirtuin 1 activator, increases IL-6 production by peripheral blood mononuclear cells of patients with knee osteoarthritis. Clin Epigenetics. 2013;5:10. doi: 10.1186/1868-7083-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesinger A, Peters W, Chappell D, Kentrup D, Reuter S, Pavenstädt H, Oberleithner H, Kümpers P. Nanomechanics of the endothelial glycocalyx in experimental sepsis. PLoS ONE. 2013;8:e80905. doi: 10.1371/journal.pone.0080905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F, de Villiers WJ, McClain CJ, Varilek GW. Green tea polyphenols block endotoxin-induced tumor necrosis factor-production and lethality in a murine model. J Nutr. 1998;128:2334–2340. doi: 10.1093/jn/128.12.2334. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Anoopkumar-Dukie S, Arora D, Davey AK. Review of the anti-inflammatory effect of SIRT1 and SIRT2 modulators on neurodegenerative diseases. Eur J Pharmacol. 2020;867:172847. doi: 10.1016/j.ejphar.2019.172847. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request