Figure 6. Tentative model of oleic acid binding to Cytoglobin.
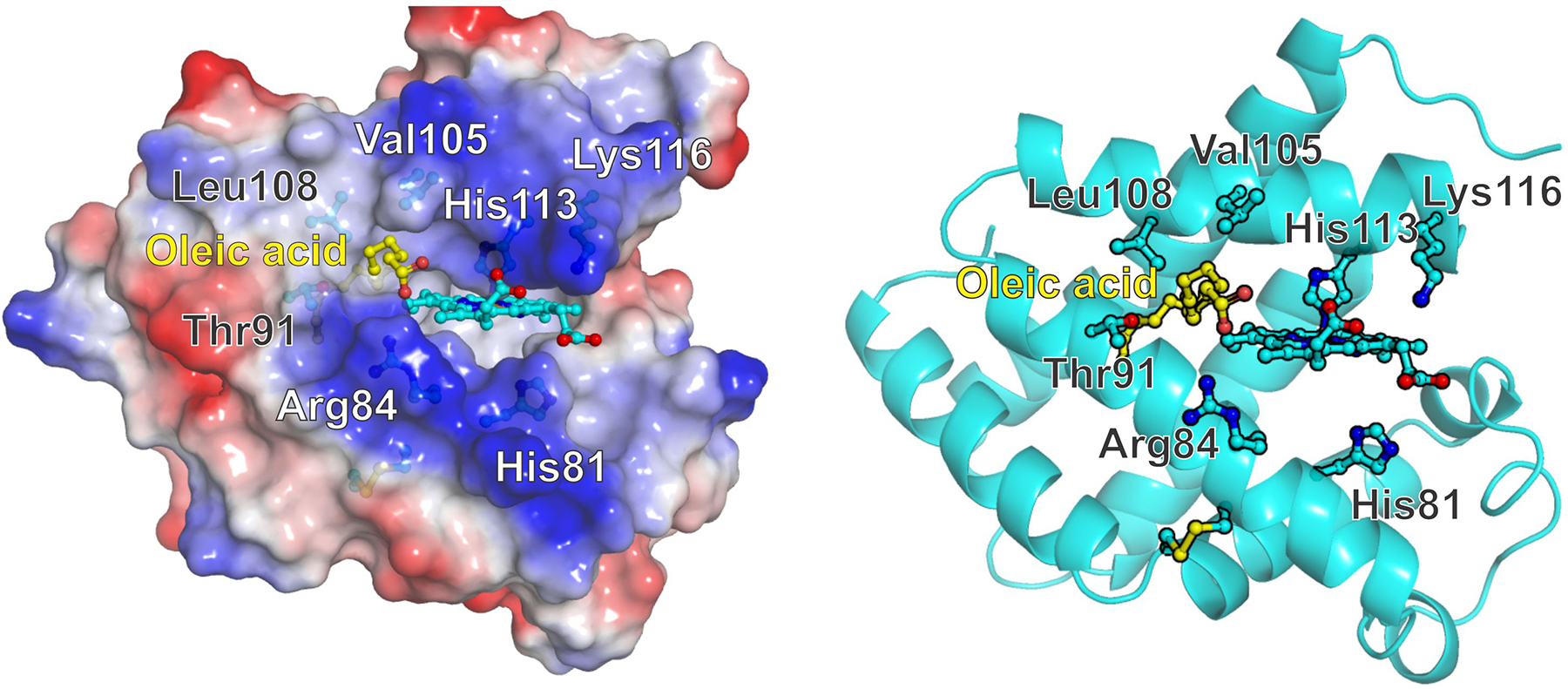
Left, surface potential view indicating the hydrophobic region around residues Val105 and Leu108 and the positive charges contributed by Arg84 and Lys116 sidechains. Right, Cartoon view showing the side residues studied in this work. The formation of the disulfide bridge between Cys38 and Cys83 promotes a movement of the E helix -containing His81- that opens a hydrophobic cavity in the proximal side of the heme. The cavity opening is surrounded by residues Arg 84, Thr91, Val105, Leu108 and Ala 112. The hydrophobic moiety of the fatty acid occupies the hydrophobic cavity whereas the carboxylate can interact with Arg84. Protein structures and electrostatic surface potentials were generated with PyMOL [52].
