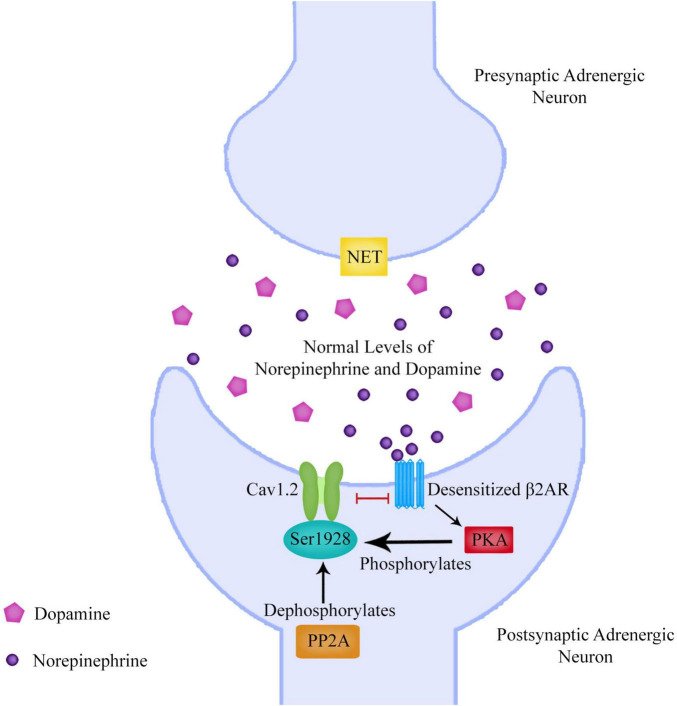
FIGURE 5.
The beta-2 adrenergic receptor (β2AR) can upregulate the L-type calcium channel Cav1.2 to allow calcium entry into the neuron. Protein Kinase A (PKA) phosphorylation of the Cav1.2 residue S1928 displaces the β2AR from Cav1.2 upon β2AR stimulation rendering Cav1.2 refractory for several minutes from further β2AR stimulation. This desensitization process is an important mechanism to prevent cellular over-excitation. Protein phosphatase 2 (PP2A) is required for reversal of PKA-mediated Cav1.2 channel phosphorylation. This means that PKA as well as PP2A are important for Cav1.2 regulation by phosphorylation and dephosphorylation of Ser1928. As PP2A dephosphorylation of Ser1928 would result in reversing the displacement of Cav1.2 from β2ARs, a dysregulation of PP2A could lead to the inability of β2ARs to resensitize.