|
|
Grouping based on HER2 (dual probe) ISH result 1. Group 1: POSITIVE, HER2/CEP17 ratio ≥2.0 AND mean HER2 copy number per cell ≥4.0 |
| 2. Group 2: HER2/CEP17 ratio ≥2.0 AND mean HER2 copy number per cell <4.0. Considered positive only if IHC is 3+ |
| 3. Group 3: HER2/CEP17 ratio per cell <2.0 AND mean HER2 copy number ≥6.0. Considered positive only if IHC is 2+ or 3+ |
| 4. Group 4: HER2/CEP17 ratio <2.0 AND mean HER2 copy number per cell ≥4.0 but <6.0. Considered positive only if IHC is 3+ |
| 5. Group 5: NEGATIVE, HER2/CEP17 ratio <2.0 AND mean HER2 copy number per cell <4.0 |
|
ISH groups
|
Biology
|
HER2/CEP17 ratio
|
Mean HER2 copy number
|
2018 ASCO/CAP recommendation
|
| 1 |
Classical HER2-amplified tumour |
≥2 |
≥4 |
Positive |
| 2 |
Chromosome 17 monosomy |
≥2 |
<4 |
Negative (HER2-low if IHC 1+/2+; 76) unless HER2 IHC is 3+d
|
| 3 |
Co-amplification (previously chromosome 17 polysomy) |
<2 |
≥6 |
Negative (HER2-low if IHC 1+; 76); unless HER2 is IHC 2+ or 3+ |
| 4 |
Borderline/uncertain |
<2 |
≥4 and <6 |
Negative (HER2-low, if IHC 1+/2+; 76) unless HER2 is IHC 3+ |
| 5 |
Classical HER2 non-amplified tumour |
<2 |
<4 |
Negative (HER2-low, if IHC 1+/2+ (76) |
| Summary of ASCO/CAP HER2 Professional Recommendation of 2018. |
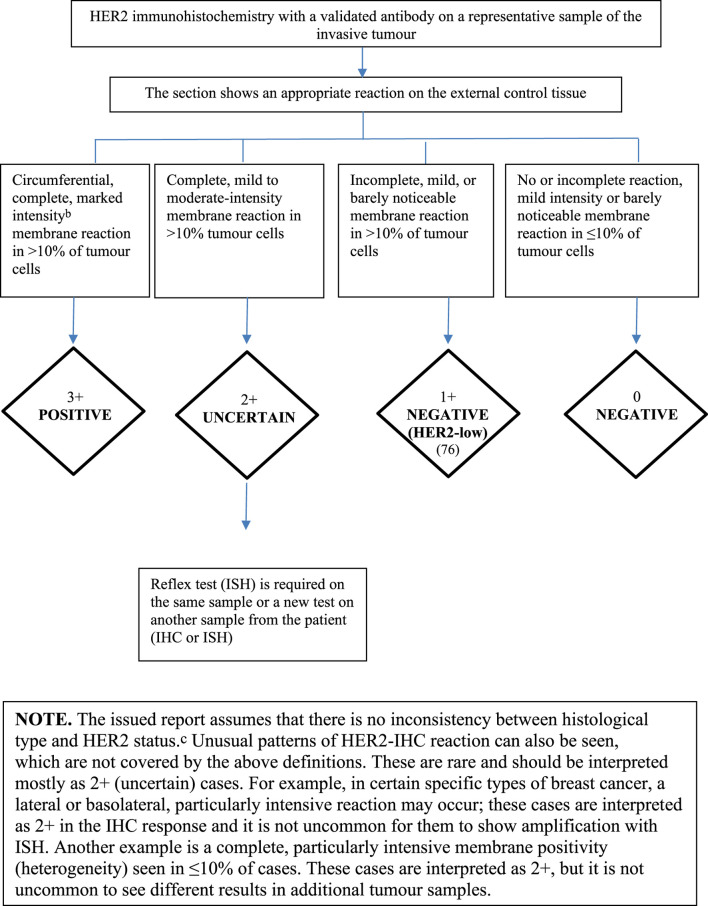
| Cases rated 3+ are considered positive for targeted treatment, while those rated 2+ are considered uncertain, including cases showing strong membrane staining in <10% of cells. Cases rated 0 and 1+ should be considered negative. (F)ISH: this is mandatory in cases of uncertain HER2 status with IHC. |
| HER2-low category encompasses non-amplified IHC 1+ and 2+ cases, and accordingly the “non-positive” cases of ISH groups 2, 3 and 4 (76). |
|
aBased on the latest (2018) ASCO/CAP recommendations (ASCO/CAP). |
|
bClearly visible at low magnification in a homogeneous, contiguous tumour cell population. |
|
cHER2 positivity is virtually non-existent in the following tumour types: |
| Histological grade 1 NST carcinomas Classical lobular carcinoma, oestrogen and progesterone receptor positive Tubular carcinoma Mucinous carcinoma Cribriform carcinoma Adenoid cystic carcinomadIn the case of HER2 monosomy, there is clinical evidence, based on retrospective analysis, that these may respond to targeted treatment in the same way as HER2 positive tumours, suggesting that targeted treatment should be considered for this group (75). HER2 testing should be performed on the surgical specimen in the following cases, even if this has previously been done on the core biopsy specimen: if the core biopsy sample contained a small amount of tumour tissue or the invasive component of the tumour was visible only in the surgical specimen. if the surgical specimen shows a high grade carcinoma not seen in the core biopsy specimen, or morphological heterogeneity or a different additional tumour nodule that was not represented by the core biopsy (30). if it is suspected that a preanalytical error has occurred during the processing of the core biopsy sample. if the HER2 assessment in the core biopsy sample yielded an uncertain result if HER2 positivity in the core biopsy sample was heterogeneous in a tumour remaining after neoadjuvant treatment.For recurrent or metastatic tumours, HER2 assessment should be repeated. Heterogeneity of HER2Definition of heterogeneity: an aggregated cell population consisting of amplified cells that make up >10% of tumour cells in the section examined. Individual amplified cells present in a mosaic-like, scattered distribution do not fall into this category. Cases as defined above are rare. Amplified and non-amplified areas should be examined separately, and HER2 / CEP17 ratio and mean HER2 copy number per cell in the two cell populations should be reported separately. The proportion of the amplified tumour cell population should be specified in the report. Cases with non-amplified and amplified areas should be considered HER2-positive. In the event of morphological heterogeneity, it is recommended to repeat HER2 testing on the surgical material (74). Pathology departments performing predictive immunohistochemical tests are expected to participate in an external quality assurance programme and achieve appropriate qualification. |

