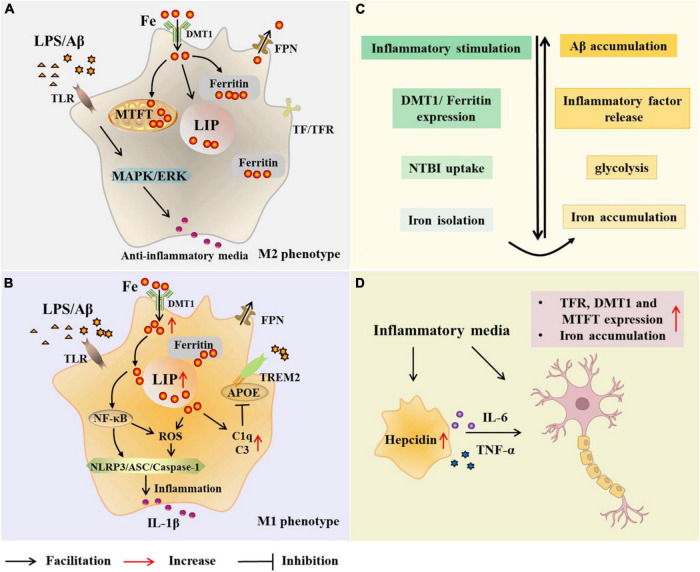
FIGURE 2.
Microglial modulations on iron metabolism in Alzheimer’s disease (AD). In the case of AD, microglia are exposed to elevated iron levels, LPS, as well as the extracellular Aβ released from damaged neurons. The expression of DMT1 and ferritin is up-regulated in M2 microglia, which preferentially increases NTBI uptake, expands iron storage in ferritin and mitochondrial ferritin, and isolates extracellular and intracellular iron (A,C). With high iron intake, microglia LIP increases, and iron and its induced ROS activate inflammasome, releasing inflammatory factors and promoting neuroinflammation (B). Iron upregulates C3 and C1q expression and inhibits the APOE-TREM2 axis, leading to reduced phagocytosis of Aβ plaques (B). Iron accumulation increases TNF-α expression and glycolysis, and reduces microglial phagocytosis (C). Microglia alter neuronal iron homeostasis in neuroinflammation. Increased hepcidin in microglia and released inflammatory factors promote iron accumulation in neurons by affecting iron uptake and storage (D). AD, Alzheimer’s disease; Aβ,amyloid beta-protein; LPS, lipopolysaccharide; TLR, toll-like receptor; DMT1, divalent metal transporter 1; FPN, ferropoiin; C1q, complement 1q; TfR, transferrin receptor; NTBI, non-transferrin-bound iron; MTFT, mitochondrial ferritin; IFN-γ, interferon γ; NF-κB, nuclearfactor-kappa B; ROS, reactive oxygen species; MAPK, mitogen activated protein kinase; ERK, extracellular regulated protein kinases; APOE, apolipoprotein E; TREM2 triggering receptor expressed on myeloid cells 2; ASC, apoptosis-associated speck-like protein containing a CARD; NLRP3, NLR family pyrin domain containing 3; TNF-α, tumor necrosis factor-α; IL-6, interleukin-6.