Abstract
Taurine (2-aminoethanesulfonic acid) is a non-essential amino acid mainly obtained through diet in humans. Despite the lack of research on the health effects of taurine in animals and humans, it is widely used as a dietary supplement. Evidence from human and animal studies indicates that taurine is involved in conjugation of bile acids and regulation of blood pressure and has anti-oxidative, anti-inflammatory, and anti-obesogenic properties. Taurine can benefit both human and non-human animal health in multiple ways. However, few interventional and epidemiological studies regarding the beneficial impacts of taurine in humans and other animals have been conducted. Here, we review the evidence from animal and human studies showing that taurine protects against dyslipidemia, obesity, hypertension, and diabetes mellitus.
Keywords: Taurine, Metabolic syndrome, Obesity, Dyslipidemia, Hypertension, Diabetes mellitus
INTRODUCTION
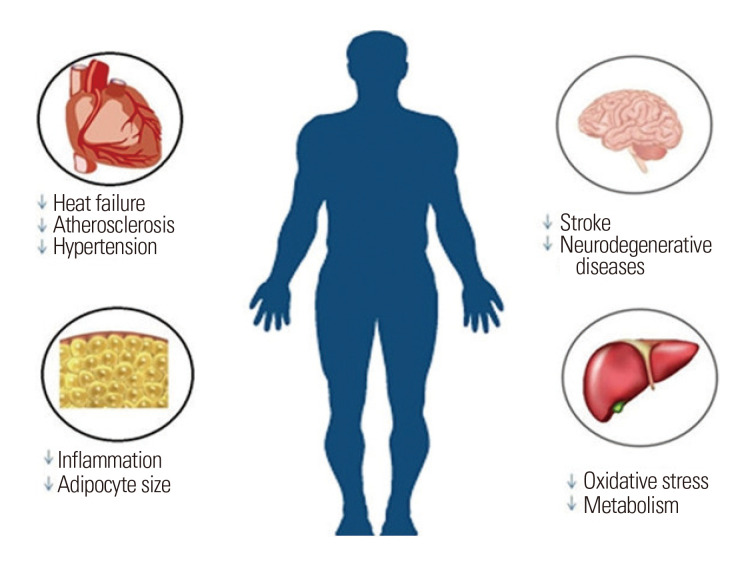
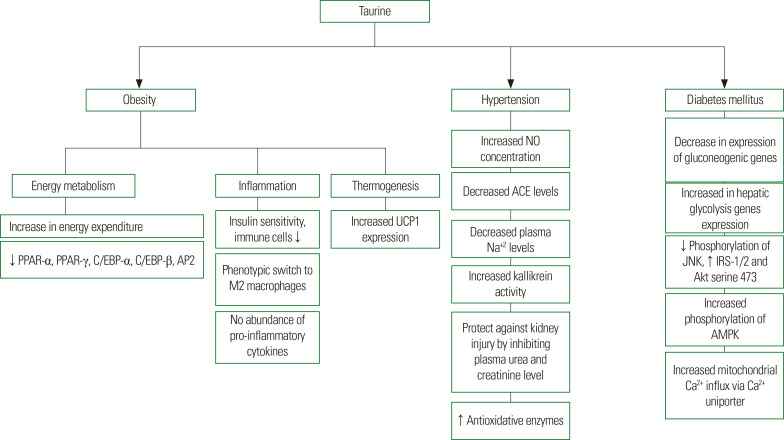
Taurine (2-aminoethanesulfonic acid), first discovered as a component of ox bile, has recently been recognized as a potential pharmaconutrient due to its various clinically significant effects in non-human animal and human models.1 Its significance in human nutrition was realized when taurine level was found to be below the normal range in the plasma and urine of preterm formula-fed infants.2,3 Taurine is considered a non-essential amino acid in rodents, an essential amino acid in cats, and a conditionally essential amino acid in humans.4 Several in vivo studies utilizing different experimental models have demonstrated the significance of taurine during development; its scarcity during various developmental phases has been linked to various pathological issues such as retardation, cardiomyopathy, and retinal degeneration.5,6 Taurine is involved in a number of metabolic processes, including osmoregulation, membrane stabilization, modulation of cellular calcium level, and detoxification.7,8 Taurine-transporter-deficient mice are characterized by impairment of various physiological functions, suggesting a crucial role for taurine in cellular homeostasis, as shown in Fig. 1.9 Moreover, taurine has been used to treat cystic fibrosis, Alzheimer disease, cardiovascular diseases, epilepsy, muscular degradation, and hepatic disorders.10 In this article, we review the sources and synthesis of taurine and examine evidence from in vitro and in vivo studies regarding the ability of taurine to protect against dyslipidemia, obesity, hypertension, and diabetes mellitus, as shown in Fig. 2.
Figure 1.
Beneficial effects of taurine in humans.
Figure 2.
Mechanisms underlying the beneficial health effects of taurine. ↓, decrease; ↑, increase; PPAR, peroxisome proliferator-activated receptor; C/EBP, CCAAT/enhancer- binding protein; AP2, adipocyte protein 2; UCP, uncoupling protein; NO, nitric oxide; ACE, angiotensin-converting enzyme; IRS, insulin receptor substrate; AMPK, AMP-activated protein kinase.
SOURCES OF TAURINE
In humans, taurine is mostly obtained through diet.11 Taurine is present in high concentrations in mussels, clams, shellfish, turkey, and dark chicken meat. Cooking adversely affects taurine level due to loss during boiling or basting in a water environment; however, taurine is retained during baking or frying processes, which are associated with minimal water loss.12
SYNTHESIS OF TAURINE
Taurine in the human body is synthesized endogenously from cysteine and methionine in the liver.13 Despite this endogenous production, taurine is mostly obtained through diet. Dietary obtained taurine is delivered to the portal vein and ultimately to the liver and blood. Taurine exists in free form in the cytoplasm in various organs and tissues such as the heart, retina, developing brain, and blood.14 Taurine is taken up by cells via taurine transporters that are highly sensitive to intracellular taurine concentration.
TAURINE AND DYSLIPIDEMIA
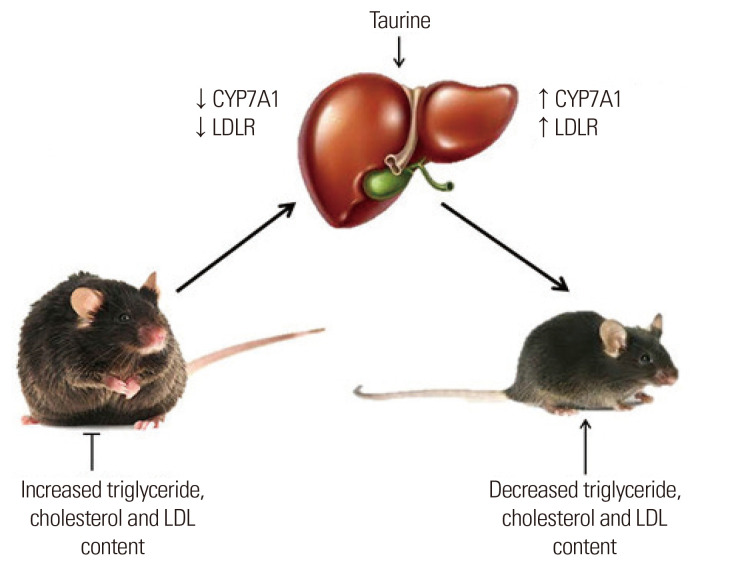
The effects of taurine on blood lipid profiles have been investigated in animal studies.15,16 Taurine consumption (1% in diet) for 8 weeks significantly reduced serum triglyceride (TG), total cholesterol (TC), and low-density lipoprotein cholesterol (LDL-C) levels as well as the LDL-C to high-density lipoprotein cholesterol (HDL-C) ratio in male Sprague-Dawley rats, as illustrated in Fig. 3.15 Taurine administration in golden Syrian hamsters fed a high-fat, high-cholesterol diet (1% or 2%) for 2 weeks significantly reduced plasma TC and TG levels.16 Taurine decreased elevated blood lipid levels and hepatic damage caused by a high-fat diet in hamsters through upregulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase, LDL receptors, and cholesterol 7 alpha-hydroxylase (CYP7A1) expression together with down regulation of serum aspartate aminotransferase, alanine aminotransferase (ALT), and C-reactive protein (CRP) levels. In addition, taurine supplementation (1% in drinking water) for 60 days significantly reduced serum TC, non–HDL-C, and TG levels but increased HDL-C level in hyperlipidemia atherosclerosis-prone quails fed a high-cholesterol diet.17 When Otsuka Long-Evans Tokushima Fatty (OLETF) rats with and without diabetes mellitus were fed a diet containing 2% and 5% taurine for 12 weeks and 9 weeks, respectively, taurine supplementation significantly decreased TG, TC, HDL-C, and LDL-C levels in both groups.18 Amelioration of dyslipidemia was due to increased insulin sensitivity and leptin modulation in OLETF rats with long-term diabetes.
Figure 3.
Taurine and dyslipidemia. CYP7A1, cholesterol 7-hydroxylase; LDLR, low-density lipoprotein receptor; LDL, low-density lipoprotein.
Taurine might attenuate dyslipidemia in animal models by preventing obesity. Taurine supplementation (2.5% in drinking water) for 70 days significantly reduced the weight of retroperitoneal and epidydimal fat pads in male Wistar rats who received subcutaneous injections of monosodium glutamate (MSG).19 Furthermore, taurine can alter the expression of genes involved in cholesterol metabolism.20 Taurine diet (5%) supplemented with either corn oil or coconut oil administered for 28 days to ovariectomized rats decreased plasma TC level due to an increase in mRNA expression of the LDL-C receptor and cholesterol CYP7A1 activity in the liver.20
Several randomized trials have been conducted to analyze the effect of taurine supplementation on the lipid profiles of humans. In a single-blind study on 22 healthy Japanese volunteers, the effects of 6 g taurine supplementation on lipid profiles were analyzed in comparison to a placebo group.21 All volunteers ate a high-cholesterol diet for three weeks; those in the control group had significant increases in TC and LDL-C levels, whereas those that received taurine showed a smaller and not significant increase in cholesterol levels compared to the control placebo group, as shown in Table 1.21-26
Table 1.
Human studies assessing the association between taurine and various diseases
| Study | Study design | Dosage and intervention | Beneficial effect |
|---|---|---|---|
| Mizushima et al. (1996)21 | 18–29-Year-old male adults (n = 22); randomized control trial (Japan) | High-cholesterol diet accompanied by 6 g of taurine or placebo/day for 3 weeks; Evaluation of total cholesterol, LDL-C, and norepinephrine levels | Taurine supplementation reduced total cholesterol and LDL-C levels. Moreover, it significantly reduced norepinephrine level in the taurine-treated group compared to the placebo group. |
| Liu et al. (2001)23 | Han (n = 775), Tibetan (n = 125), Kazak (n = 204), Uygur (n = 510) subjects aged between 49–54 years; cross-sectional study | NA Correlation of taurine excretion with blood pressure |
A positive correlation between decreased DBP and taurine supplementation was seen in Han and Tibetan subjects, whereas no such correlation was evident in Uygur and Kazak subjects. |
| Milei et al. (1992)25 | 12 Subjects with angina aged 30–60 years; randomized controlled trial | 5 g taurine or placebo 1–3 hours before CABG Evaluation of oxidative stress |
Ratio of reperfusion and pre-ischemic sample means was significantly reduced in the taurine group (1.12) compared with the placebo group (2.45) |
| Mizushima et al. (1997)24 | 433 Subjects from Japan and 269 subjects from Brazil aged 45–59 years; cross-sectional study | NA Evaluation of hypercholesterolemia hypertension |
Hypercholesterolemia prevalence was significantly different between Japanese (5.8%) and Brazilian (28.3%) men, while hypertension was significantly different between Japanese women (14%) and Brazilian women (32.0%); this was attributed to higher consumption of fish high in taurine by Japanese residents. |
| Rosa et al. (2014)26 | 16 Obese and 8 normal weight women (n = 24); randomized double-blind placebo-controlled study | Placebo with 3 g/day starch flour or 3 g/day taurine supplementation | Taurine supplementation restored taurine level in the obese group and decreased markers of inflammation |
| Zhang et al. (2004)22 | Overweight and obese college students (n = 30) aged 20–22 years; double-blind randomization | Taurine 3 g/day or placebo taken orally for 7 weeks | Taurine-supplemented group showed decrease in body weight, TG content, and AI index |
LDL-C, low-density lipoprotein cholesterol; NA, not applicable; DBP, diastolic blood pressure; CABG, coronary artery bypass graft; TG, triglyceride; AI, atherogenic index.
TAURINE AND OBESITY
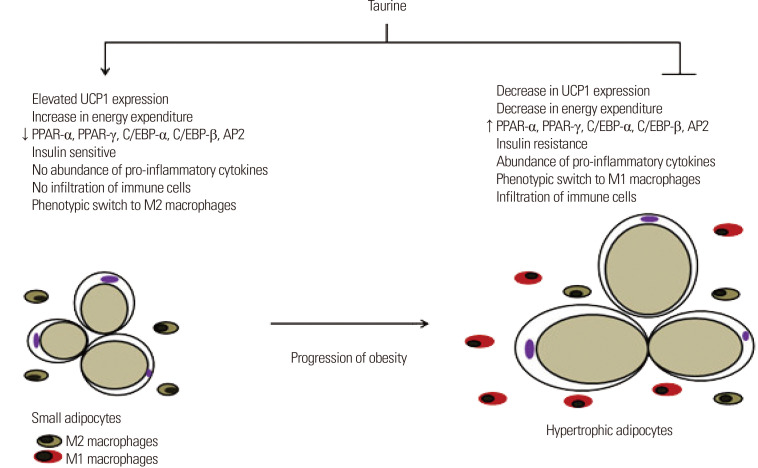
Taurine has anti-obesity effects in various animal models.27 Taurine supplementation (2% in drinking water) for 28 weeks resulted in decreased expression of adipogenic genes, including peroxisome proliferator-activated receptor (PPAR)-α, PPAR-γ, CCAAT/enhancer-binding protein (C/EBP)-α, C/EBP-β, and adipocyte protein 2 (AP2) in the white adipose tissue (WAT) of high-fat diet-fed ICR mice as shown in Fig. 4.27 In another study, taurine supplementation (2.5% in drinking water) for 100 days significantly decreased body weight with less retroperitoneal and perigonadal fat weight gain in male obese rats injected with MSG.28 The anti-obesity effects of taurine might result from lower food/caloric intake.29,30 Mice administered 2% taurine in their drinking water for 10 weeks gained less weight on a high-fat diet and showed significantly lower visceral fat weight than control mice.30 In that study, mice administered taurine showed decreased food intake.
Figure 4.
Taurine and obesity. ↓, decrease; ↑, increase; UCP, uncoupling protein; PPAR, peroxisome proliferator-activated receptor; C/EBP, CCAAT/enhancer-binding protein; AP2, adipocyte protein 2.
Taurine also might attenuate obesity by altering energy metabolism.31 Mice fed a taurine-containing diet (5%) for 18 weeks had lower body weight, parametrial WAT weight, percentage body fat, and adipocyte size than control mice.31 The anti-obesity effects of taurine were mediated by an increase in resting oxygen consumption in the light phase. As depicted in Fig. 4, the increase in resting oxygen consumption might have resulted in part from an increase in expression of genes involved in energy expenditure such as PPARγ coactivator 1α (PGC1α), PPARα, PPARγ, nuclear respiratory factor 2α, lipoprotein lipase, acyl-coenzyme A (CoA) oxidase, acyl-CoA synthetase, medium-chain acyl-CoA dehydrogenase (MCAD), the β-subunit of adenosine triphosphate (ATP) synthetase, and uncoupling protein (UCP) in WAT. Moreover, taurine treatment increased the expression of genes involved in adipocyte browning and fatty acid oxidation including PGC1α, UCP1, mitochondrial cytochrome c (Cyc), mitochondrial transcription factor A (mtTFA), carnitine palmitoyl-CoA transferase 1β (Cpt1β), and MCAD in inguinal WAT. Furthermore, protein levels of PGC1α and UCP1 in inguinal WAT were elevated by taurine treatment. The taurine-induced elevation of UCP1 protein level in the inguinal WAT of mice was confirmed by immunohistochemistry. Together, these results indicate that taurine has anti-obesity properties mediated in part by increased energy expenditure.
The anti-obesity effects of taurine might prevent chronic inflammation in adipose tissue.32 Taurine supplementation (2.5% in drinking water) significantly decreased retroperitoneal and perigonadal fat pad weight in MSG-injected obese rats.32 These rats showed increased phosphorylation of inhibitor κB-α in retroperitoneal adipose tissue, which was decreased by taurine supplementation. The anti-inflammatory effects of taurine chloramine (TauCl) are associated with inhibition of nuclear factor κB pathway activation.33 TauCl, through inhibition of the signal transducer and activator of transcription 3 (STAT-3) pathway, has been shown to control the expression of adiponectin, interleukin 6 (IL-6), leptin, and IL-8 in differentiated human adipocytes.34 Moreover, TauCl (0–400 μM) inhibited the expression of CD11, IL-18, CD86, and IL-1β, all of which are M1 macrophage markers, in lipopolysaccharides/tumor necrosis factor alpha (TNF-α) activated bone marrow-derived macrophages. Moreover, the expression of TNF-α was significantly reduced by TauCl in a dose-dependent manner.35 These studies suggest that taurine and TauCl attenuate obesity-associated inflammation.
Taurine affects differentiation,36 lipolysis,37 and energy expenditure of adipocytes.31 TauCl treatment inhibited the differentiation of preadipocytes into adipocytes36 through a reduction in protein levels of PPAR-γ, C/EBP-α, and sterol regulatory element binding protein 1 (SREBP1). Taurine treatment increased lipolysis in isolated rat adipocytes stimulated with isoproterenol.37 Furthermore, taurine treatment of C3H10T1/2 adipocytes increased the expression of genes related to energy expenditure and thermogenesis such as PGC1α, UCP1, mtTFA, and carnitine palmitoyltransferase-1 beta (Cpt1β),31 in addition to increasing the basal oxygen consumption rate of C3H10T1/2 adipocytes. Knockdown of PGC1α in C3H10T1/2 adipocytes decreased taurine-induced expression of UCP1, mtTFA, and Cpt1β as well as protein levels of PGC1α and UCP1.
Mechanisms underlying the anti-obesity effects of taurine have been less well explored in humans than in non-human animal models. A randomized double-blind trial of 30 obese college students was conducted to study the anti-obesity effects of taurine.22 After 7 weeks of taurine supplementation (3 g/day), overall weight and plasma TG content were significantly reduced in the taurine group compared to the placebo group. A randomized, double-blind, placebo-controlled study of 16 obese women and 8 non-obese women who received taurine supplementation (3 g/day) for 8 weeks revealed an increase in plasma taurine (+97%) and adiponectin levels (+12%) in the taurine-supplemented group.26 Moreover, this group showed decrease in the inflammatory marker CRP (–29%) and the lipid peroxidation marker thiobarbituric acid reactive substances (–20%) compared to the control group.
In a placebo-controlled trial of 12 patients25 with stable angina, they received 5 g of taurine by intravenous infusion 1–3 hours before coronary bypass surgery. This supplementation reduced the levels of lipid peroxidation products during reperfusion and restored blood flow. In preoperative biopsy samples, the taurine pretreated group had a reperfusion to oxidative stress ratio of 1.12 compared to 2.45 in the placebo group. Kim and Cha38 suggested that taurine acts as an anti-inflammatory agent by neutralizing neutrophil oxidants and TauCl, a byproduct formed during reaction between taurine and hypochlorous acid. This impedes the inflammatory process as summarized in Table 2.38-42 In healthy mitochondria, a conjugate of taurine is formed by its reaction with the uridine residue of transfer RNA (tRNALeu(UUR)). However, in some mitochondrial diseases, this conjugate is not formed and results in suppression of the expression of certain mitochondrial encoded proteins, including nicotinamide adenine dinucleotide hydrogen (NADH)-ubiquinone oxidoreductase chain 6 (ND6). Scarcity of ND6 decreases generation of ATP by mitochondria.43 During oxidative stress, permeabilization of the inner membrane of mitochondria occurs, and taurine disrupts the associated chain of events.44 Taurine supplementation can replenish taurine level for taurine reactions, restoring normal mitochondrial function and protein synthesis and reducing superoxide generation. Moreover, taurine protects sensitive antioxidative enzymes by decreasing the formation of reactive oxygen species (ROS).45 Obesity is closely linked with non-alcoholic fatty liver disease (NAFLD), which is caused by an increase in intrahepatic TGs with or without inflammation and fibrosis (steatohepatitis). A study conducted by Obinata et al.46 of 10 children with NAFLD revealed that taurine supplementation improved serum ALT level and decreased the ratios of glycine/taurine-conjugated bile acids. These results indicate that taurine supplementation can be an effective treatment for NAFLD.46
Table 2.
Clinical studies of the mechanisms underlying taurine’s beneficial effects on human health
| Study | Beneficial effect | Mechanism |
|---|---|---|
| Kim and Cha38, Marcinkiewicz and Kontny39 (2014) | Anti-oxidative effects | Anti-inflammation by neutralization of hypochlorous acid to produce taurine chloramine |
| Schaffer et al. (2016)40 | Energy metabolism | Activates complex l and NADH-sensitive enzymes by reducing the NADH/NAD+ ratio during glycolysis |
| Kadooka et al. (2010)41 | Endoplasmic reticulum stress | Ameliorates brain injury during stroke by inhibiting ER stress |
| Ramila et al. (2015)42 | Ca2+ homeostasis | Taurine depletion leads to cardiomyopathy due to reduced ER Ca2+ ATPase activity |
NADH, nicotinamide adenine dinucleotide hydrogen; ER, endoplasmic reticulum; ATPase, aenosine tri phosphate synthetase.
TAURINE AND HYPERTENSION
Taurine is considered to have protective effects against hypertension based on evidence from several animal studies.47,48 Rats with taurine deficiency induced by β-alanine treatment had more severe hypertension than control counterparts on an 8% NaCl diet for 6 weeks followed by uninephrectomy.47 Male Sprague-Dawley rats fed a 35% fructose diet with 5% fructose in drinking water for 4 weeks had significantly increased body weight and systolic blood pressure than control rats, but this was ameliorated by taurine administration (2% taurine in drinking water). The decrease in blood pressure was attributed to increased plasma nitric oxide (NO) concentration; NO lowers blood pressure by dilating blood vessels.48 In male Wistar rats treated with nandrolone decanoate (10 mg/kg body weight) by intragluteal injection once a week for 12 weeks to induce hypertension, the increase in systolic blood pressure was prevented by taurine administration (2% taurine in drinking water).49 The nandrolone decanoate-induced increase in systolic blood pressure mediated by induction of plasma angiotensin-converting enzyme (ACE) activity was abolished by taurine treatment. Moreover, when male Wistar rats were exposed to electric foot shocks and noise for 2 hr/day for 20 days, the combined stress significantly increased tail artery blood pressure, but this was inhibited by intragastric administration of taurine (200 mg/kg/day), which decreased ACE level and enhanced the formation of NO.50 As hypertension is associated with impaired NO production,51 restoring NO generation from nitrites and nitrates is considered to be beneficial for treatment of hypertension.
In addition to taurine’s ability to modulate ACE and NO synthesis, it might prevent hypertension by altering the kallikrein-kinin system.52 The Kallikrein-kinin system consists of kallikreins, kiniogens, kinins, kinin-degrading enzymes, and kinin receptors.53 Kallikreins are a family of serine proteases that produce kinins from precursor kininogens. Kinins are hydrolyzed and inactivated by kininases, including ACE.54 The kallikrein-kinin system plays an important role in blood pressure.55 An increase in blood pressure in high-fructose-fed rats was prevented by taurine administration (2% in drinking water).52 Kallikrein activity in the heart, kidney, plasma, and urine was significantly reduced by high fructose consumption but was increased by taurine administration in rats. Plasma sodium level was reduced by taurine in high-fructose-diet-fed rats due to increased urinary excretion of sodium.
In hypertension, taurine might play an important role in preventing kidney injury.56 Male Wistar rats were orally administered N-nitro L-arginine-methyl-ester (L-NAME), an NO synthase inhibitor, at 40 mg/kg bodyweight for 14 days.56 Rats then received oral taurine (200 mg/kg body weight), which significantly reduced systolic, diastolic, and mean arterial blood pressure. It also increased renal NO level in L-NAME-treated hypertensive rats. Taurine consumption inhibited the induction of plasma levels of urea and creatinine by L-NAME, indicating prevention of renal injury in L-NAME-treated rats.
An inverse correlation between urinary excretion of taurine and blood pressure in humans was reported by the WHO Cardiovascular Diseases and Alimentary Comparison (WHO-CARDIAC).57 A negative correlation between 24-hour taurine excretion and both systolic and diastolic blood pressure was reported in the Kazak population. In the 775 Han Chinese subjects, a significant negative correlation between 24-hour taurine excretion and diastolic blood pressure was noted, while similar negative correlations were found between 24-hour taurine excretion and both systolic and diastolic blood pressure in the 125 Tibetan participants.23
In a cross-sectional study of 433 middle-aged Japanese subjects and 269 Japanese immigrants in Brazil, the native Japanese showed significantly higher urinary excretion of taurine than the Japanese immigrants. The authors of that study concluded that the prevalence of hypertension and hypercholesterolemia was lower in native Japanese subjects than Japanese immigrants living in Brazil, suggesting that the environmental factor of taurine intake rather than genetics was a key factor behind underlying hypertension and hypercholesterolemia prevalence.24
A double-blind, placebo-controlled study was conducted for a period of one week in 19 hypertensive patients (aged 20–25 years).58 Taurine supplementation (6 g/day) significantly decreased epinephrine level and systolic and diastolic blood pressure compared to the placebo group but had no effect on norepinephrine level. Dietary habits play a major role in heart health, and an adequate intake of legumes, vegetables, fruits, and whole grains is strongly recommended based on research findings to prevent coronary artery diseases.59,60 In a double-blind randomized study involving 30 overweight or obese college students (body mass index >25 kg/m2), subjects received 3 g taurine for 7 weeks, and lipid profiles were compared between baseline and 7 weeks. The taurine-supplemented group showed a significant decrease (P<0.04) in plasma TG (>8 mg/dL) level compared with the placebo group (8 mg/dL).61 Moreover, the taurine-supplemented group showed a significant reduction in atherogenic index (2.75–2.30) after seven weeks compared to the placebo group (2.91–2.99). These findings suggest that taurine supplementation can reduce TG level. However, given the limitations of this study, including its small sample size, the short length of supplementation, and lack of health status examination, further large-scale studies are needed to confirm these study findings.
ANTI-OXIDATIVE EFFECTS OF TAURINE
The protective effects of taurine on kidney injury are potentially mediated by its antioxidant capacity.56 L-NAME-treated rats had significantly higher myeloperoxidase activity as well as hydrogen peroxide and malondialdehyde levels in the kidney, all of which were reduced by taurine. The antioxidant effect of taurine was mediated by an increase in the activities of antioxidant enzymes, including superoxide dismutase (SOD), catalase, and glutathione peroxidase, in the kidney. Consistently, taurine treatment increased the level of reduced glutathione in the rat kidney. In addition, taurine treatment (1% in drinking water) for 4 weeks significantly reduced systolic blood pressure in high-salt (8% NaCl) diet-fed Dahl salt-sensitive rats.62
Taurine also has antioxidant properties in the aorta.63 Male Wistar rats fed a low-protein (6%) diet for 90 days showed significant increases in systolic, diastolic, and mean arterial blood pressure, which were significantly attenuated by taurine supplementation (2.5% in drinking water).63 Taurine decreased ROS production and increased NO bioavailability in aortic sections in low-protein diet-fed rats. The antioxidative effects of taurine were mediated by a decrease in the protein level of p47phox and an increase in the protein levels of manganese SOD and extracellular SOD in the rat aorta. Taurine-treated rats showed improved aortic morphometric parameters such as decreased cross-sectional area and wall/lumen ratio.
TAURINE AND DIABETES MELLITUS
Studies have shown that taurine has beneficial effects in animal models of diabetes mellitus.64 Male C57BL/6J mice fed a high-fat diet for 8 weeks that received 5% taurine supplementation in their drinking water64 showed improved glucose tolerance as measured by the intraperitoneal glucose tolerance test (ipGTT) compared to control group mice. In another animal study, male Wistar rats fed a high-fructose diet (60%) and that received 2% taurine in their drinking water for 30 days65 showed a significant attenuation in the high fructose-induced increase in plasma glucose, fructosamine, glycated protein, and glycated hemoglobin levels. In another study, hyperglycemia was induced by a 6-hour infusion of 50% dextrose through catheters inserted into the internal jugular vein of male Sprague-Dawley rats to maintain the plasma glucose level at 15 mM.66 Taurine infusion (0.35 mg/kg/min) with 50% dextrose significantly increased the glucose infusion rate and glucose utilization as measured by hyperinsulinemic-euglycemic clamp in these rats. Taurine supplementation also reduced the expression of gluconeogenic genes such as glucose 6-phosphatase, fructose 1,6-bisphosphate, forkhead box protein O1, phosphoenolpyruvate carboxykinase, and PGC1α in the livers of As2O3-treated mice. In addition, taurine increased the hepatic expression of genes involved in glycolysis such as glucokinase and L type pyruvate kinase. Moreover, taurine increased the protein expression of glucose transporter 2 and the phosphorylation of glycogen synthase and decreased phosphorylation of glycogen synthase kinase‐3β in the rat liver. These results indicate that taurine can rectify hepatic glucose metabolism in an insulin-resistant state.
Taurine has also been shown to improve hepatic insulin signaling.67,68 Male Wistar rats infused with 20% intralipids and 20 U/mL heparin with taurine (0.35 mg/kg/min) at 5.5 μL/min for 48 hours67 showed an increased glucose infusion rate during hyperinsulinemic-euglycemic clamp with reduced hepatic glucose production. Taurine-supplemented rats showed improved insulin signaling with reduced phosphorylation of JNK, insulin receptor substrate 1/2 (IRS-1/2), and serine phosphorylation and increased insulin-stimulated IRS-1/2 tyrosine phosphorylation and Akt serine 473 phosphorylation in the liver. In another animal study, male C57BL/6 mice that received 5% taurine in drinking water for 45 days and who were then fed a high-fat diet (34% of fat) for 60 days together with taurine supplementation showed improved glucose tolerance as measured by ipGTT.68 Taurine also increased phosphorylation of phosphatase and tensin homolog (PTEN) and Akt in the liver, which might enhance insulin signaling.
In addition, taurine ameliorated insulin signaling in skeletal muscle in diabetes mellitus.69,70 Male Wister rats fed a high-sugar and high-fat diet for 8 weeks to develop insulin resistance then received 2% taurine supplementation in their drinking water for 4 weeks to evaluate the therapeutic effect of taurine on insulin resistance.69 Inorganic arsenic exposure is a risk factor for type 2 diabetes mellitus.71 In the skeletal muscle of mice exposed to As2O3, phosphorylation of glycogen synthase kinase increased, and phosphorylation of IRS and Akt decreased; taurine inhibited these changes. As2O3-induced ectopic lipid accumulation in mouse skeletal muscle was prevented by taurine, which was mediated in part by a reduction in the protein level of CD36 in skeletal muscle.71 In addition, taurine attenuated the increase in protein expression of LC3-II and p62 in skeletal muscle, indicating that taurine inhibits autophagy and improves As2O3-induced insulin resistance in skeletal muscle. When L6 myoblast cells were treated with taurine (100 μM), they showed enhanced glucose uptake both in the presence and absence of 100 nM of insulin.70 The effect of taurine on cellular glucose uptake was mediated via the AMP-activated protein kinase (AMPK) pathway, as the induction of glucose uptake by taurine was significantly lowered by the AMPK inhibitor compound C. Consistently, taurine treatment increased phosphorylation of AMPK in L6 myotubes.
Furthermore, taurine has protective effects on pancreatic beta cells.72,73 Male Swiss mice were fed a high-fat diet containing 36% saturated fat with or without taurine supplementation (5% in drinking water) for 19 weeks.72 Taurine administration prevented the high-fat diet-induced increase in plasma glucose, insulin, pancreatic beta cell mass, and islet mass. In UCP-overexpressing beta cells isolated from rat pancreas, 3 mM taurine treatment significantly increased insulin secretion in response to 10 mM glucose.74 Elevated expression of UCP2 in pancreatic beta cells by long-term exposure to high glucose or acid levels is associated with impaired glucose-induced insulin secretion.59 Taurine elevated methyl pyruvate-induced mitochondrial Ca2+ in mitochondria isolated from UCP2-overexpressing insulin-secreting beta cells.73 These results suggest that taurine improves glucose sensitivity potentially by increasing mitochondrial Ca2+ influx via+uniporters, improving mitochondrial metabolism in pancreatic beta cells.
Taurine affects glucose homeostasis in humans by moderating beta cell insulin secretion, insulin signaling pathways, and/or post-receptor events.75-77 Taurine deficiency has been linked to the severity of diabetes mellitus and associated complications.78 In the study of Sak et al.,79 plasma taurine level was lower in type 2 diabetic subjects than control subjects, and the authors of that study suggested a correlation between low taurine level and diabetic neuropathy. Oxidative stress and diabetic complications have also been shown to be associated with taurine.79 Thus, taurine can be preventative and play a therapeutic role in the context of diabetes mellitus.80,81 Brownlee82 established an association between generation of ROS and severity of diabetic complications due to generation of superoxide anions in glucose-treated mitochondria. A deficiency of taurine in diabetic conditions worsens and enhances oxidative stress.82 Taurine depletion results in the formation of defective tRNAs whose translation results in defective proteins and subsequent defective assembly complexes involved in the respiratory chain. This translates into reduced transport of electrons through the electron transport chain and the consequent formation of superoxide anions instead of the generation of oxygen.83 Suzuki et al.81 confirmed that taurine forms macromolecular complexes and is not simply present as a free amino acid by utilizing mutant mitochondria tRNA taurine-containing uridine residues (Leu and Lys) in the phagocytic cells of mitochondrial encephalomyopathy patients, mitochondrial encephalopathy, lactic acidosis, and stroke-like episodes (MELAS) and myoclonic epilepsy with ragged-red fibers (MERRF).84 Moreover, taurine’s anti-oxidative properties reduced hypochlorous acid by attenuating the bleomycin-mediated up-regulation of inducible nitric oxide synthetase (iNOS).85 According to Wu et al.,86 taurine prevents calcium accumulation and overload by disrupting the sequence of events that leads to calcium overload, mitochondrial damage, and further ROS formation. These findings suggest a key role for taurine in reducing oxidative stress damage and the complications of diabetes mellitus.
CONCLUSION
Animal and human studies have suggested several plausible mechanisms through which taurine reduces the risks of obesity, dyslipidemia, hypertension, and diabetes mellitus. Nutritional studies of dietary sources of taurine and its bioavailability in animals and humans need to be conducted to obtain more definitive answers regarding the influence of long-term taurine level on various diseases.
Footnotes
CONFLICTS OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
Study concept and design: MB and JEY; acquisition of data: MB and KA; drafting of the manuscript: KA and MB; critical revision of the manuscript: MB and JEY; and study supervision: JEY.
REFERENCES
- 1.Guta RC. Is taurine a pharmaconutrient. J Pharmacol Ther Res. 2018;2:18–20. [Google Scholar]
- 2.Wright CE, Tallan HH, Lin YY, Gaull GE. Taurine: biological update. Annu Rev Biochem. 1986;55:427–53. doi: 10.1146/annurev.bi.55.070186.002235. [DOI] [PubMed] [Google Scholar]
- 3.Seidel U, Huebbe P, Rimbach G. Taurine: a regulator of cellular redox homeostasis and skeletal muscle function. Mol Nutr Food Res. 2019;63:e1800569. doi: 10.1002/mnfr.201800569. [DOI] [PubMed] [Google Scholar]
- 4.Lourenço R, Camilo ME. Taurine: a conditionally essential amino acid in humans?: an overview in health and disease. Nutr Hosp. 2002;17:262–70. [PubMed] [Google Scholar]
- 5.Chen C, Xia S, He J, Lu G, Xie Z, Han H. Roles of taurine in cognitive function of physiology, pathologies and toxication. Life Sci. 2019;231:116584. doi: 10.1016/j.lfs.2019.116584. [DOI] [PubMed] [Google Scholar]
- 6.Sturman JA, Gaull GE. Taurine in the brain and liver of the developing human and monkey. J Neurochem. 1975;25:831–5. doi: 10.1111/j.1471-4159.1975.tb04414.x. [DOI] [PubMed] [Google Scholar]
- 7.Kendler BS. Taurine: an overview of its role in preventive medicine. Prev Med. 1989;18:79–100. doi: 10.1016/0091-7435(89)90056-X. [DOI] [PubMed] [Google Scholar]
- 8.Jo HG, Kim MJ, Moon BY, Cheong SH. Antioxidant and laxative effects of taurine-xylose, a synthetic taurine-carbohydrate derivative, in loperamide-induced constipation in Sprague-Dawley rats. J Exerc Nutrition Biochem. 2019;23:6–13. doi: 10.20463/jenb.2019.0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Warskulat U, Flögel U, Jacoby C, Hartwig HG, Thewissen M, Merx MW, et al. Taurine transporter knockout depletes muscle taurine levels and results in severe skeletal muscle impairment but leaves cardiac function uncompromised. FASEB J. 2004;18:577–9. doi: 10.1096/fj.03-0496fje. [DOI] [PubMed] [Google Scholar]
- 10.Manabe S, Kurroda I, Okada K, Morishima M, Okamoto M, Harada N, et al. Decreased blood levels of lactic acid and urinary excretion of 3-methylhistidine after exercise by chronic taurine treatment in rats. J Nutr Sci Vitaminol (Tokyo) 2003;49:375–80. doi: 10.3177/jnsv.49.375. [DOI] [PubMed] [Google Scholar]
- 11.Redmond HP, Stapleton PP, Neary P, Bouchier-Hayes D. Immunonutrition: the role of taurine. Nutrition. 1998;14:599–604. doi: 10.1016/S0899-9007(98)00097-5. [DOI] [PubMed] [Google Scholar]
- 12.Spitze AR, Wong DL, Rogers QR, Fascetti AJ. Taurine concentrations in animal feed ingredients; cooking influences taurine content. J Anim Physiol Anim Nutr (Berl) 2003;87:251–62. doi: 10.1046/j.1439-0396.2003.00434.x. [DOI] [PubMed] [Google Scholar]
- 13.Wen C, Li F, Zhang L, Duan Y, Guo Q, Wang W, et al. Taurine is involved in energy metabolism in muscles, adipose tissue, and the liver. Mol Nutr Food Res. 2019;63:e1800536. doi: 10.1002/mnfr.201800536. [DOI] [PubMed] [Google Scholar]
- 14.Hayes KC, Sturman JA. Taurine in metabolism. Annu Rev Nutr. 1981;1:401–25. doi: 10.1146/annurev.nu.01.070181.002153. [DOI] [PubMed] [Google Scholar]
- 15.Jo HG, Kim MJ, Moon BY, Cheong SH. Antioxidant and laxative effects of taurine- xylose, a synthetic taurine-carbohydrate derivative, in loperamide-induced constipation in Sprague-Dawley rats. J Exerc Nutrition Biochem. 2019;23:6–13. doi: 10.20463/jenb.2019.0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takenaga T, Imada K, Otomo S. Hypolipidemic effect of taurine in golden Syrian hamsters. Adv Exp Med Biol. 2000;483:187–92. doi: 10.1007/0-306-46838-7_20. [DOI] [PubMed] [Google Scholar]
- 17.Murakami S, Sakurai T, Tomoike H, Sakono M, Nasu T, Fukuda N. Prevention of hypercholesterolemia and atherosclerosis in the hyperlipidemia- and atherosclerosis-prone Japanese (LAP) quail by taurine supplementation. Amino Acids. 2010;38:271–8. doi: 10.1007/s00726-009-0247-6. [DOI] [PubMed] [Google Scholar]
- 18.Kim KS, Oh DH, Kim JY, Lee BG, You JS, Chang KJ, et al. Taurine ameliorates hyperglycemia and dyslipidemia by reducing insulin resistance and leptin level in Otsuka Long-Evans Tokushima fatty (OLETF) rats with long-term diabetes. Exp Mol Med. 2012;44:665–73. doi: 10.3858/emm.2012.44.11.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nardelli TR, Ribeiro RA, Balbo SL, Vanzela EC, Carneiro EM, Boschero AC, et al. Taurine prevents fat deposition and ameliorates plasma lipid profile in monosodium glutamate-obese rats. Amino Acids. 2011;41:901–8. doi: 10.1007/s00726-010-0789-7. [DOI] [PubMed] [Google Scholar]
- 20.Kishida T, Miyazato S, Ogawa H, Ebihara K. Taurine prevents hypercholesterolemia in ovariectomized rats fed corn oil but not in those fed coconut oil. J Nutr. 2003;133:2616–21. doi: 10.1093/jn/133.8.2616. [DOI] [PubMed] [Google Scholar]
- 21.Mizushima S, Nara Y, Sawamura M, Yamori Y. Effects of oral taurine supplementation on lipids and sympathetic nerve tone. Adv Exp Med Biol. 1996;403:615–22. doi: 10.1007/978-1-4899-0182-8_68. [DOI] [PubMed] [Google Scholar]
- 22.Zhang M, Izumi I, Kagamimori S, Sokejima S, Yamagami T, Liu Z, et al. Role of taurine supplementation to prevent exercise-induced oxidative stress in healthy young men. Amino Acids. 2004;26:203–7. doi: 10.1007/s00726-003-0002-3. [DOI] [PubMed] [Google Scholar]
- 23.Liu L, Liu L, Ding Y, Huang Z, He B, Sun S, et al. Ethnic and environmental differences in various markers of dietary intake and blood pressure among Chinese Han and three other minority peoples of China: results from the WHO Cardiovascular Diseases and Alimentary Comparison (CARDIAC) Study. Hypertens Res. 2001;24:315–22. doi: 10.1291/hypres.24.315. [DOI] [PubMed] [Google Scholar]
- 24.Mizushima S, Moriguchi EH, Ishikawa P, Hekman P, Nara Y, Mimura G, et al. Fish intake and cardiovascular risk among middle-aged Japanese in Japan and Brazil. J Cardiovasc Risk. 1997;4:191–9. doi: 10.1097/00043798-199706000-00006. [DOI] [PubMed] [Google Scholar]
- 25.Milei J, Ferreira R, Llesuy S, Forcada P, Covarrubias J, Boveris A. Reduction of reperfusion injury with preoperative rapid intravenous infusion of taurine during myocardial revascularization. Am Heart J. 1992;123:339–45. doi: 10.1016/0002-8703(92)90644-B. [DOI] [PubMed] [Google Scholar]
- 26.Rosa FT, Freitas EC, Deminice R, Jordão AA, Marchini JS. Oxidative stress and inflammation in obesity after taurine supplementation: a double-blind, placebo-controlled study. Eur J Nutr. 2014;53:823–30. doi: 10.1007/s00394-013-0586-7. [DOI] [PubMed] [Google Scholar]
- 27.Kim KS, Jang MJ, Fang S, Yoon SG, Kim IY, Seong JK, et al. Anti-obesity effect of taurine through inhibition of adipogenesis in white fat tissue but not in brown fat tissue in a high-fat diet-induced obese mouse model. Amino Acids. 2019;51:245–54. doi: 10.1007/s00726-018-2659-7. [DOI] [PubMed] [Google Scholar]
- 28.Bonfleur ML, Borck PC, Ribeiro RA, Caetano LC, Soares GM, Carneiro EM, et al. Improvement in the expression of hepatic genes involved in fatty acid metabolism in obese rats supplemented with taurine. Life Sci. 2015;135:15–21. doi: 10.1016/j.lfs.2015.05.019. [DOI] [PubMed] [Google Scholar]
- 29.Camargo RL, Batista TM, Ribeiro RA, Branco RC, Da Silva PM, Izumi C, et al. Taurine supplementation preserves hypothalamic leptin action in normal and protein-restricted mice fed on a high-fat diet. Amino Acids. 2015;47:2419–35. doi: 10.1007/s00726-015-2035-9. [DOI] [PubMed] [Google Scholar]
- 30.Figueroa AL, Figueiredo H, Rebuffat SA, Vieira E, Gomis R. Taurine treatment modulates circadian rhythms in mice fed a high fat diet. Sci Rep. 2016;6:36801. doi: 10.1038/srep36801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tsuboyama-Kasaoka N, Shozawa C, Sano K, Kamei Y, Kasaoka S, Hosokawa Y, et al. Taurine (2-aminoethanesulfonic acid) deficiency creates a vicious circle promoting obesity. Endocrinology. 2006;147:3276–84. doi: 10.1210/en.2005-1007. [DOI] [PubMed] [Google Scholar]
- 32.Caetano LC, Bonfleur ML, Ribeiro RA, Nardelli TR, Lubaczeuski C, do Nascimento da Silva J, et al. Taurine supplementation regulates Iκ-Bα protein expression in adipose tissue and serum IL-4 and TNF-α concentrations in MSG obesity. Eur J Nutr. 2017;56:705–13. doi: 10.1007/s00394-015-1114-8. [DOI] [PubMed] [Google Scholar]
- 33.Kanayama A, Inoue J, Sugita-Konishi Y, Shimizu M, Miyamoto Y. Oxidation of Ikappa Balpha at methionine 45 is one cause of taurine chloramine-induced inhibition of NF-kappa B activation. J Biol Chem. 2002;277:24049–56. doi: 10.1074/jbc.M110832200. [DOI] [PubMed] [Google Scholar]
- 34.Kim KS, Ji HI, Chung H, Kim C, Lee SH, Lee YA, et al. Taurine chloramine modulates the expression of adipokines through inhibition of the STAT-3 signaling pathway in differentiated human adipocytes. Amino Acids. 2013;45:1415–22. doi: 10.1007/s00726-013-1612-z. [DOI] [PubMed] [Google Scholar]
- 35.Murakami S. The physiological and pathophysiological roles of taurine in adipose tissue in relation to obesity. Life Sci. 2017;186:80–6. doi: 10.1016/j.lfs.2017.08.008. [DOI] [PubMed] [Google Scholar]
- 36.Kim KS, Choi HM, Ji HI, Kim C, Kim JY, Song R, et al. Effect of taurine chloramine on differentiation of human preadipocytes into adipocytes. Adv Exp Med Biol. 2013;775:247–57. doi: 10.1007/978-1-4614-6130-2_21. [DOI] [PubMed] [Google Scholar]
- 37.Piña-Zentella G, de la Rosa-Cuevas G, Vázquez-Meza H, Piña E, de Piña MZ. Taurine in adipocytes prevents insulin-mediated H2O2 generation and activates Pka and lipolysis. Amino Acids. 2012;42:1927–35. doi: 10.1007/s00726-011-0919-x. [DOI] [PubMed] [Google Scholar]
- 38.Kim C, Cha YN. Taurine chloramine produced from taurine under inflammation provides anti-inflammatory and cytoprotective effects. Amino Acids. 2014;46:89–100. doi: 10.1007/s00726-013-1545-6. [DOI] [PubMed] [Google Scholar]
- 39.Marcinkiewicz J, Kontny E. Taurine and inflammatory diseases. Amino Acids. 2014;46:7–20. doi: 10.1007/s00726-012-1361-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schaffer SW, Shimada-Takaura K, Jong CJ, Ito T, Takahashi K. Impaired energy metabolism of the taurine deficient heart. Amino Acids. 2016;48:549–58. doi: 10.1007/s00726-015-2110-2. [DOI] [PubMed] [Google Scholar]
- 41.Kadooka Y, Sato M, Imaizumi K, Ogawa A, Ikuyama K, Akai Y, et al. Regulation of abdominal adiposity by probiotics (Lactobacillus gasseri SBT2055) in adults with obese tendencies in a randomized controlled trial. Eur J Clin Nutr. 2010;64:636–43. doi: 10.1038/ejcn.2010.19. [DOI] [PubMed] [Google Scholar]
- 42.Ramila KC, Jong CJ, Pastukh V, Ito T, Azuma J, Schaffer SW. Role of protein phosphorylation in excitation-contraction coupling in taurine deficient hearts. Am J Physiol Heart Circ Physiol. 2015;308:H232–9. doi: 10.1152/ajpheart.00497.2014. [DOI] [PubMed] [Google Scholar]
- 43.Jong CJ, Azuma J, Schaffer S. Mechanism underlying the antioxidant activity of taurine: prevention of mitochondrial oxidant production. Amino Acids. 2012;42:2223–32. doi: 10.1007/s00726-011-0962-7. [DOI] [PubMed] [Google Scholar]
- 44.Ricci C, Pastukh V, Leonard J, Turrens J, Wilson G, Schaffer D, et al. Mitochondrial DNA damage triggers mitochondrial-superoxide generation and apoptosis. Am J Physiol Cell Physiol. 2008;294:C413–22. doi: 10.1152/ajpcell.00362.2007. [DOI] [PubMed] [Google Scholar]
- 45.Schaffer SW, Jong CJ, Ito T, Azuma J. Role of taurine in the pathologies of MELAS and MERRF. Amino Acids. 2014;46:47–56. doi: 10.1007/s00726-012-1414-8. [DOI] [PubMed] [Google Scholar]
- 46.Obinata K, Maruyama T, Hayashi M, Watanabe T, Nittono H. Effect of taurine on the fatty liver of children with simple obesity. Adv Exp Med Biol. 1996;403:607–13. doi: 10.1007/978-1-4899-0182-8_67. [DOI] [PubMed] [Google Scholar]
- 47.Mozaffari MS, Patel C, Abdelsayed R, Schaffer SW. Accelerated NaCl-induced hypertension in taurine-deficient rat: role of renal function. Kidney Int. 2006;70:329–37. doi: 10.1038/sj.ki.5001503. [DOI] [PubMed] [Google Scholar]
- 48.Rahman MM, Park HM, Kim SJ, Go HK, Kim GB, Hong CU, et al. Taurine prevents hypertension and increases exercise capacity in rats with fructose-induced hypertension. Am J Hypertens. 2011;24:574–81. doi: 10.1038/ajh.2011.4. [DOI] [PubMed] [Google Scholar]
- 49.Roşca AE, Stoian I, Badiu C, Gaman L, Popescu BO, Iosif L, et al. Impact of chronic administration of anabolic androgenic steroids and taurine on blood pressure in rats. Braz J Med Biol Res. 2016;49:e5116. doi: 10.1590/1414-431x20165116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lv Q, Dong G, Cao S, Wu G, Feng Y, Mei L, et al. Effects of taurine on blood index of hypothalamic pituitary adrenal (HPA) axis of stress-induced hypertensive rat. Adv Exp Med Biol. 2015;803:613–21. doi: 10.1007/978-3-319-15126-7_49. [DOI] [PubMed] [Google Scholar]
- 51.Taddei S, Virdis A, Mattei P, Ghiadoni L, Sudano I, Salvetti A. Defective L-arginine-nitric oxide pathway in offspring of essential hypertensive patients. Circulation. 1996;94:1298–303. doi: 10.1161/01.CIR.94.6.1298. [DOI] [PubMed] [Google Scholar]
- 52.Nandhini AT, Anuradha CV. Hoe 140 abolishes the blood pressure lowering effect of taurine in high fructose-fed rats. Amino Acids. 2004;26:299–303. doi: 10.1007/s00726-003-0003-2. [DOI] [PubMed] [Google Scholar]
- 53.Madeddu P, Emanueli C, El-Dahr S. Mechanisms of disease: the tissue kallikrein-kinin system in hypertension and vascular remodeling. Nat Clin Pract Nephrol. 2007;3:208–21. doi: 10.1038/ncpneph0444. [DOI] [PubMed] [Google Scholar]
- 54.Clements J, Hooper J, Dong Y. The human tissue kallikrein and kallikrein-related peptidase family. In: Rawlings ND, Salvesen G, editors. Handbook of proteolytic enzymes. Elsevier; London: 2013. pp. 2747–56. [DOI] [Google Scholar]
- 55.Oparil S, Zaman MA, Calhoun DA. Pathogenesis of hypertension. Ann Intern Med. 2003;139:761–76. doi: 10.7326/0003-4819-139-9-200311040-00011. [DOI] [PubMed] [Google Scholar]
- 56.Adedara IA, Alake SE, Olajide LO, Adeyemo MO, Ajibade TO, Farombi EO. Taurine ameliorates thyroid hypofunction and renal injury in L-NAME-induced hypertensive rats. Drug Res (Stuttg) 2019;69:83–92. doi: 10.1055/a-0643-4604. [DOI] [PubMed] [Google Scholar]
- 57.Yamori Y, Liu L, Ikeda K, Miura A, Mizushima S, Miki T, et al. Distribution of twenty-four hour urinary taurine excretion and association with ischemic heart disease mortality in 24 populations of 16 countries: results from the WHO-CARDIAC study. Hypertens Res. 2001;24:453–7. doi: 10.1291/hypres.24.453. [DOI] [PubMed] [Google Scholar]
- 58.Fujita T, Ando K, Noda H, Ito Y, Sato Y. Effects of increased adrenomedullary activity and taurine in young patients with borderline hypertension. Circulation. 1987;75:525–32. doi: 10.1161/01.CIR.75.3.525. [DOI] [PubMed] [Google Scholar]
- 59.Kushi LH, Byers T, Doyle C, Bandera EV, McCullough M, McTiernan A, et al. American Cancer Society Guidelines on Nutrition and Physical Activity for cancer prevention: reducing the risk of cancer with healthy food choices and physical activity. CA Cancer J Clin. 2006;56:254–81. doi: 10.3322/canjclin.56.5.254. [DOI] [PubMed] [Google Scholar]
- 60.Musaiger AO. Diet and prevention of coronary heart disease in the Arab Middle East countries. Med Princ Pract. 2002;11 Suppl 2:9–16. doi: 10.1159/000066415. [DOI] [PubMed] [Google Scholar]
- 61.Zhang M, Bi LF, Fang JH, Su XL, Da GL, Kuwamori T, et al. Beneficial effects of taurine on serum lipids in overweight or obese non-diabetic subjects. Amino Acids. 2004;26:267–71. doi: 10.1007/s00726-003-0059-z. [DOI] [PubMed] [Google Scholar]
- 62.Chiba Y, Ando K, Fujita T. The protective effects of taurine against renal damage by salt loading in Dahl salt-sensitive rats. J Hypertens. 2002;20:2269–74. doi: 10.1097/00004872-200211000-00027. [DOI] [PubMed] [Google Scholar]
- 63.Maia AR, Batista TM, Victorio JA, Clerici SP, Delbin MA, Carneiro EM, et al. Taurine supplementation reduces blood pressure and prevents endothelial dysfunction and oxidative stress in post-weaning protein-restricted rats. PLoS One. 2014;9:e105851. doi: 10.1371/journal.pone.0105851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Camargo RL, Batista TM, Ribeiro RA, Velloso LA, Boschero AC, Carneiro EM. Effects of taurine supplementation upon food intake and central insulin signaling in malnourished mice fed on a high-fat diet. Adv Exp Med Biol. 2013;776:93–103. doi: 10.1007/978-1-4614-6093-0_10. [DOI] [PubMed] [Google Scholar]
- 65.Nandhini AT, Thirunavukkarasu V, Anuradha CV. Stimulation of glucose utilization and inhibition of protein glycation and AGE products by taurine. Acta Physiol Scand. 2004;181:297–303. doi: 10.1111/j.1365-201X.2004.01287.x. [DOI] [PubMed] [Google Scholar]
- 66.Harada N, Ninomiya C, Osako Y, Morishima M, Mawatari K, Takahashi A, et al. Taurine alters respiratory gas exchange and nutrient metabolism in type 2 diabetic rats. Obes Res. 2004;12:1077–84. doi: 10.1038/oby.2004.135. [DOI] [PubMed] [Google Scholar]
- 67.Wu N, Lu Y, He B, Zhang Y, Lin J, Zhao S, et al. Taurine prevents free fatty acid-induced hepatic insulin resistance in association with inhibiting JNK1 activation and improving insulin signaling in vivo. Diabetes Res Clin Pract. 2010;90:288–96. doi: 10.1016/j.diabres.2010.08.020. [DOI] [PubMed] [Google Scholar]
- 68.Cappelli AP, Zoppi CC, Barbosa-Sampaio HC, Costa JM, Jr, Protzek AO, Morato PN, et al. Taurine-induced insulin signalling improvement of obese malnourished mice is associated with redox balance and protein phosphatases activity modulation. Liver Int. 2014;34:771–83. doi: 10.1111/liv.12291. [DOI] [PubMed] [Google Scholar]
- 69.Zhao D, Lv Q, Yang J, Wu G, Liu M, Yang Q, et al. Taurine improves lipid metabolism and skeletal muscle sensitivity to insulin in rats fed with high sugar and high fat diet. Adv Exp Med Biol. 2019;1155:133–46. doi: 10.1007/978-981-13-8023-5_12. [DOI] [PubMed] [Google Scholar]
- 70.Cheong SH, Chang KJ. Antidiabetic effect of taurine in cultured rat skeletal l6 myotubes. Adv Exp Med Biol. 2013;775:311–20. doi: 10.1007/978-1-4614-6130-2_26. [DOI] [PubMed] [Google Scholar]
- 71.Beck R, Styblo M, Sethupathy P. Arsenic exposure and type 2 diabetes: microRNAs as mechanistic links? Curr Diab Rep. 2017;17:18. doi: 10.1007/s11892-017-0845-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ribeiro RA, Santos-Silva JC, Vettorazzi JF, Cotrim BB, Mobiolli DD, Boschero AC, et al. Taurine supplementation prevents morpho-physiological alterations in high-fat diet mice pancreatic β-cells. Amino Acids. 2012;43:1791–801. doi: 10.1007/s00726-012-1263-5. [DOI] [PubMed] [Google Scholar]
- 73.Han J, Bae JH, Kim SY, Lee HY, Jang BC, Lee IK, et al. Taurine increases glucose sensitivity of UCP2-overexpressing beta-cells by ameliorating mitochondrial metabolism. Am J Physiol Endocrinol Metab. 2004;287:E1008–18. doi: 10.1152/ajpendo.00008.2004. [DOI] [PubMed] [Google Scholar]
- 74.Lee SH, Lee HY, Kim SY, Lee IK, Song DK. Enhancing effect of taurine on glucose response in UCP2-overexpressing beta cells. Diabetes Res Clin Pract. 2004;66 Suppl 1:S69–74. doi: 10.1016/j.diabres.2003.08.018. [DOI] [PubMed] [Google Scholar]
- 75.Zheng Y, Ceglarek U, Huang T, Wang T, Heianza Y, Ma W, et al. Plasma taurine, diabetes genetic predisposition, and changes of insulin sensitivity in response to weight-loss diets. J Clin Endocrinol Metab. 2016;101:3820–6. doi: 10.1210/jc.2016-1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Santos-Silva JC, Ribeiro RA, Vettorazzi JF, Irles E, Rickli S, Borck PC, et al. Taurine supplementation ameliorates glucose homeostasis, prevents insulin and glucagon hypersecretion, and controls β, α, and δ-cell masses in genetic obese mice. Amino Acids. 2015;47:1533–48. doi: 10.1007/s00726-015-1988-z. [DOI] [PubMed] [Google Scholar]
- 77.De la Puerta C, Arrieta FJ, Balsa JA, Botella-Carretero JI, Zamarrón I, Vázquez C. Taurine and glucose metabolism: a review. Nutr Hosp. 2010;25:910–9. [PubMed] [Google Scholar]
- 78.Das J, Roy A, Sil PC. Mechanism of the protective action of taurine in toxin and drug induced organ pathophysiology and diabetic complications: a review. Food Funct. 2012;3:1251–64. doi: 10.1039/c2fo30117b. [DOI] [PubMed] [Google Scholar]
- 79.Sak D, Erdenen F, Müderrisoglu C, Altunoglu E, Sozer V, Gungel H, et al. The relationship between plasma taurine levels and diabetic complications in patients with type 2 diabetes mellitus. Biomolecules. 2019;9:96. doi: 10.3390/biom9030096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Szymański K, Winiarska K. Taurine and its potential therapeutic application. Postepy Hig Med Dosw (Online) 2008;62:75–86. [PubMed] [Google Scholar]
- 81.Suzuki T, Suzuki T, Wada T, Saigo K, Watanabe K. Novel taurine-containing uridine derivatives and mitochondrial human diseases. Nucleic Acids Res Suppl. 2001;(1):257–8. doi: 10.1093/nass/1.1.257. [DOI] [PubMed] [Google Scholar]
- 82.Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005;54:1615–25. doi: 10.2337/diabetes.54.6.1615. [DOI] [PubMed] [Google Scholar]
- 83.Franconi F, Di Leo MA, Bennardini F, Ghirlanda G. Is taurine beneficial in reducing risk factors for diabetes mellitus? Neurochem Res. 2004;29:143–50. doi: 10.1023/B:NERE.0000010443.05899.2f. [DOI] [PubMed] [Google Scholar]
- 84.Suzuki T, Suzuki T, Wada T, Saigo K, Watanabe K. Taurine as a constituent of mitochondrial tRNAs: new insights into the functions of taurine and human mitochondrial diseases. EMBO J. 2002;21:6581–9. doi: 10.1093/emboj/cdf656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gurujeyalakshmi G, Wang Y, Giri SN. Suppression of bleomycin-induced nitric oxide production in mice by taurine and niacin. Nitric Oxide. 2000;4:399–411. doi: 10.1006/niox.2000.0297. [DOI] [PubMed] [Google Scholar]
- 86.Wu H, Jin Y, Wei J, Jin H, Sha D, Wu JY. Mode of action of taurine as a neuroprotector. Brain Res. 2005;1038:123–31. doi: 10.1016/j.brainres.2005.01.058. [DOI] [PubMed] [Google Scholar]