FIGURE 2.
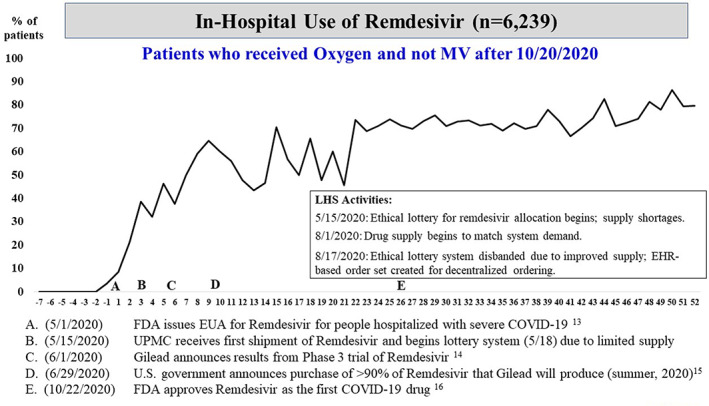
Plot of weekly prevalence (%) of in‐hospital use of remdesivir among patients who received oxygen (but not mechanical ventilation after October 20, 2020). On the x‐axis, negative numbers reflect weeks prior to seminal event “A,” the date (May 1, 2020) in which the Food and Drug Administration (FDA) issued Emergency Use Authorization (EUA) for remdesivir for patients hospitalized with severe coronavirus disease 2019 (COVID‐19)
