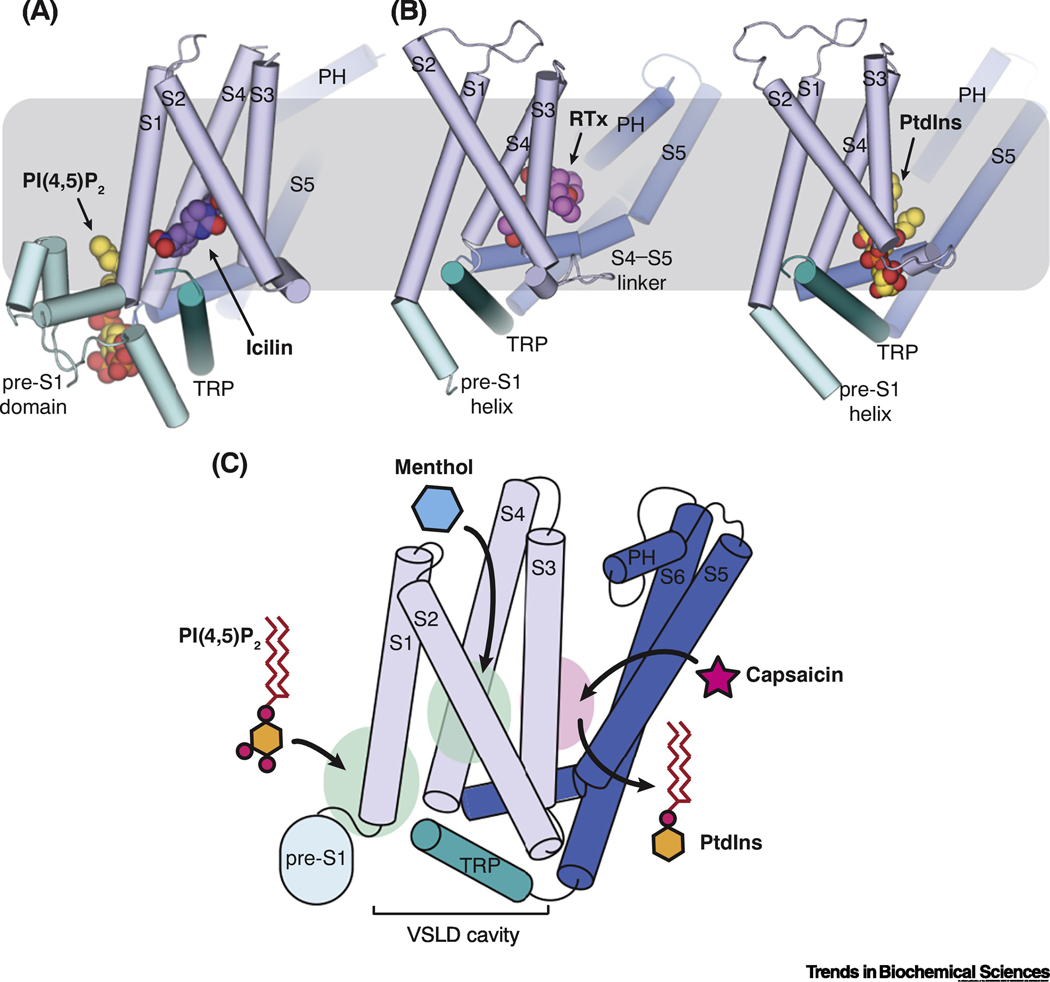
Figure 6. The design principle of synergetic actions of PI(4,5)P2 and cooling agonists on TRPM8 activation.
(A and B) Comparison of the distinct binding sites for lipid and agonists in TRPM8 (A) and TRPV1 (B) channels. The gray box represents the plasma membrane. Cytoplasmic domains of the channels are simplified.
(C) Schematic diagram depicting a discrete binding site for menthol in the VSLD cavity and the strategic position of PI(4,5)P2 binding for allosteric coupling with cooling agonist in TRPM8. In contrast, capsaicin and membrane lipid share the same binding pocket above the S4-S5 linker in TRPV1, where PtdIns is proposed to act as a competitive antagonist for vanilloid activation based on the structure. The locations representative of ligand binding in TRPM8 and TRPV1 are shown as green and pink ovals, respectively. Cytoplasmic domains are omitted for simplicity.