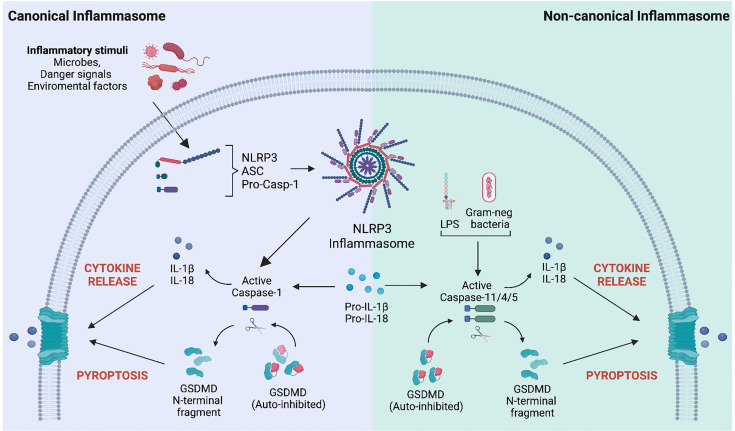
Figure 1.
Mechanisms of Gasdermin D activation following canonical and non-canonical inflammasome stimulation. Left. Canonical inflammasome activation is a well-structured process involving several steps. Upon sensing specific inflammatory stimuli (microbes, danger signals, and environmental factors), the inflammasome sensor (i.e., NOD-like receptor protein 3, NLRP3) recruits the precursor of caspase-1 (pro-caspase-1) via the protein adaptor apoptosis-associated speck-like protein containing a CARD (ASC). Upon inflammasome complex formation, pro-caspase-1 is activated by autocatalytic processing and converts the pro-IL-1β and pro-IL-18 precursors into their bioactive forms. Matured caspase-1 also cleaves the pore-forming protein gasdermin D (GSDMD), generating the N-terminal domain of GSDMD (GSDMD-NT), which relocates to the plasma membrane and oligomerizes to form a pore. Pore formation allows the release of mature IL-1β and IL-18 and drives a type of inflammatory cell death known as pyroptosis. Right. The non-canonical inflammasome pathway is activated by intracellular sensing of Gram-negative bacteria or lipopolysaccharides (LPS). It involves the activation of human caspase 4/5 or caspase-11 in mice. Active caspases directly process the pro-IL-1β and pro-IL-18 precursors and GSDMD. Like the canonical inflammasome activation mode, GSDMD pores allow the passive release of IL-1β/IL-18 cytokines and pyroptosis.