Abstract
Sleep deprivation (SD) is known to impair hippocampus‐dependent memory processes, in part by stimulating the phosphodiesterase (PDE) activity. In the present study, we assessed in mice whether SD also affects spatial pattern separation, a cognitive process that specifically requires the dentate gyrus (DG) subregion of the hippocampus. Adult male mice were trained in an object pattern separation (OPS) task in the middle of the light phase and then tested 24 hr thereafter. In total, we conducted three studies using the OPS task. In the first study, we validated the occurrence of pattern separation and tested the effects of SD. We found that 6 hr of SD during the first half of the light phase directly preceding the test trial impaired the spatial pattern separation performance. As a next step, we assessed in two consecutive studies whether the observed SD‐induced performance deficits could be prevented by the systemic application of two different PDE inhibitors that are approved for human use. Both the PDE4 inhibitor roflumilast and PDE5 inhibitor vardenafil successfully prevented SD‐induced deficits in spatial pattern separation. As a result, these PDE inhibitors have clinical potential for the prevention of memory deficits associated with loss of sleep.
Keywords: dentate gyrus, memory, pattern separation, roflumilast, sleep deprivation, sleep disorders, vardenafil

1. INTRODUCTION
Memory is the storage of information over time (McGaugh, 2000). Some of these bits of information can be highly similar or even overlapping. To be able to distinguish between overlapping information, the brain uses pattern separation, which is commonly defined as the ability to form separate representations from highly similar, yet slightly different events or stimuli (Yassa & Stark, 2011). Pattern separation occurs in the dentate gyrus (DG), a subregion of the hippocampus (Dillon et al., 2017; Yassa & Stark, 2011). Deficits in this process are among the first and most severe symptoms observed during aging and in disorders characterized by memory dysfunction such as Alzheimer's disease, post‐traumatic stress disorder, and schizophrenia.
Hippocampus‐dependent memory formation is promoted by sleep and disrupted by sleep deprivation (SD; Abel, Havekes, Saletin, & Walker, 2013; Kreutzmann, Havekes, Abel, & Meerlo, 2015). The negative impact of SD on hippocampal function is at least partly caused by increased phosphodiesterase (PDE) activity and related suppression of cAMP signaling (Havekes et al., 2014; Havekes, Park, Tolentino, et al., 2016; Vecsey et al., 2009). Because cGMP and cAMP signaling potentially target the same downstream signaling molecules known to be affected by SD (i.e., CREB (Wong, Tann, Ibanez, & Sajikumar, 2019), LIMK1‐cofilin (Havekes, Park, Tudor, et al., 2016; Wong et al., 2019; Zulauf et al., 2009)), cGMP signaling may also be a relevant therapeutic target in this respect. While disrupting effects of SD have been reported for a variety of hippocampal tasks (Havekes & Abel, 2017; Havekes, Meerlo, & Abel, 2015), its impact on pattern separation remains to be defined.
The latter becomes even more interesting as neuronal connectivity in the DG itself is disrupted by sleep loss (Meerlo, Mistlberger, Jacobs, Heller, & McGinty, 2009; Raven, Meerlo, Van der Zee, Abel, & Havekes, 2018). Moreover, sleep loss is a common feature of the aforementioned disorders characterized by deficits in pattern separation (e.g., Kheirbek, Klemenhagen, Sahay, & Hen, 2012; Van Erum, Van Dam, & De Deyn, 2019; van Goethem, van Hagen, & Prickaerts, 2018; van Os & Kapur, 2009).
Therefore, the first aim of the current study was to investigate the effect of SD in a well‐validated mouse model for spatial pattern separation. To this end, 12 male 57BL/6J mice (Charles River) were ordered at 6 weeks of age and pair‐housed. One week before the start of the experiments when the animals were 8–16 weeks old, animals were individually housed. The experimental room was kept under constant temperature (22°) and a 12 h light/12 h dark cycle (lights on 9:00–21:00). Poly carb clear cages with a stainless steel wired lid were provided with nesting material, cardboard rolls, and sawdust bedding. Chow diet and water were available ad libitum. All procedures were approved by the national Central Authority for Scientific Procedures on Animals (CCD) and the Institutional Animal Welfare Body (IvD, University of Groningen, The Netherlands), and conform Directive 2010/63/EU. Prior to the studies, the animals were handled daily for 3 days, adapted to the procedures, and allowed to explore the empty arena for 5 min on 2 separate days.
We first confirmed the occurrence of spatial pattern separation with the object pattern separation (OPS) task (Figure 1a). The OPS task is a novel, translational spatial pattern separation paradigm highly sensitive to cognitive impairment and extensively described elsewhere (van Goethem et al., 2018). Briefly, the OPS paradigm consists of two trials. During the learning trial (T1), the rectangular arena contains two identical objects placed (and oriented) symmetrically on a horizontal line, in which the mouse is allowed to explore for 10 min (Figure 1a). The arena is made of PVC and has a length of 40 cm, width of 30 cm, and height of 50 cm high. The four walls of the arena consist of grey colored PVC and the bottom consists of transparent PVC. Four pairs of two identical objects are used. These objects are either two blue aluminum cylinders (height 12 cm and diameter 3.5 cm), two orange aluminum cylinders with tapering tops (height 12 cm and diameter at widest point 3.5 cm), two green glass cylinders (height 12 cm and diameter 2.5 cm), or two pink round vases (height 10 cm and diameter ranging from 3.5 cm at the bottom to 1.5 at the top). Inside the arena, two spatial cues are presented at opposite sides at the short walls of the rectangular arena. One cue consists of black and white striping, while the other cue consists of a black and white checkerboard pattern. After a 24 hr interval, mice are placed back in the apparatus for the test trial of 10 min (T2). During T2, one of the two similar objects is randomly displaced along a straight line on one of the five possible locations in either direction according to the randomization and location scheme. In other words, during T2, mice are confronted with a new spatial arrangement. The distance between two adjacent positions is always 3.75 cm. This means that during the test trial (T2) the object is moved 0 cm for position 1; 1 × 3.75 cm for position 2; 2 × 3.75 cm for position 3; 3 × 3.75 cm for position 4; 4 × 3.75 cm for position 5. All positions are 7.5 cm away from the vertical walls and position 5 (which is closest to the horizontal wall is still 5 cm away from the horizontal wall). Experimenters were blind to treatment conditions during testing and scoring. Pattern separation is scored as the relative time spent on the displaced object. Usually, mice show a good pattern separation performance when the displacement is maximal (Figure 1, “position 5”) (van Goethem et al., 2018). However, when the displacement of the object is subtler (Figure 1, “position 3”), the task becomes more difficult increasing the need for pattern separation.
Figure 1.
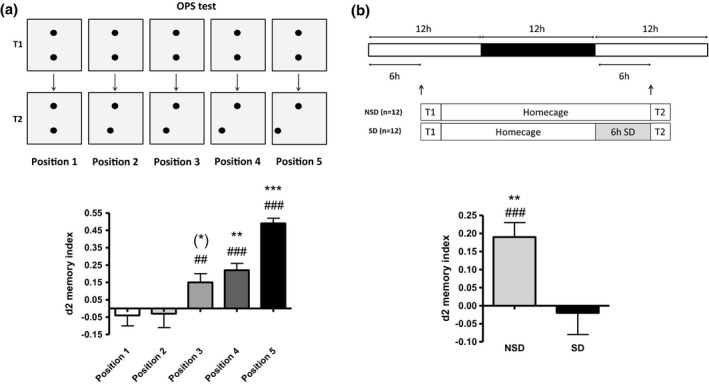
(a) Spatial pattern separation performance (d2 values as mean + SEM) as measured with the OPS test (24 h interval; # represents significant difference from zero (## = p < .01, ### = p < .001) by independent samples t test; * indicates a significant difference from position 1 (** = p < .01, *** = p < .001, and * = p = .051, by paired samples t test; N = 12). (b) Six hours of SD at the beginning of the light phase directly preceding T2 impairs spatial pattern separation performance in the OPS (position 3; 24 h interval; # represents a significant difference from zero (### = p < .001), by independent samples t test; * represents a significant difference between NSD and SD (** = p < .01) by paired samples t test; N = 12). NSD = non‐sleep deprived; SD = sleep deprived.
In line with previous work, we showed that reducing the moved distance of the relocated object (position 5 to 1) decreases the discrimination score (d2), indicating that the task became increasingly difficult (Figure 1). Position 3 showed to be the minimal distinguishable distance (different from zero (chance level) but not from position 1), which suggests a maximal DG activation (van Goethem et al., 2018; van Goethem, Schreiber, Newman‐Tancredi, Varney, & Prickaerts, 2015). The latter is important, as this position truly requires pattern separation and not merely spatial memory (a point often overlooked in rodent studies). As a result, position 3 was used during the test trial (T2) in the consecutive studies.
As a next step, we tested the effects of SD on spatial pattern separation (Figure 1b). Mice were trained 6 hr into the light phase and left undisturbed for approximately 18 hr. Thereafter, mice were kept awake for 6 hr at the beginning of the light phase when sleep pressure is highest and directly preceding the test trial (T2). The latter was done to maximize the effect of SD on pattern separation as, in the current setup, during T2 the new information (i.e., the relocation of one object) has to be integrated and properly distinguished from the old information (i.e., original object position during T1). SD before or after T1 (as commonly observed in most other mnemonic and sleep studies (e.g., (Havekes, Park, Tudor, et al., 2016)) would affect the acquisition and consolidation of the old information. This can affect subsequent pattern separation during T2, but would not directly test the effect of SD on spatial pattern separation itself. Mice were sleep deprived using the gentle stimulation method as described previously (Havekes, Park, Tudor, et al., 2016). Importantly, we did not use any objects, cages, clean bedding, or other arousing stimuli to keep the animals awake to avoid confounding effects of arousal during SD. This SD method has been validated previously using EEG recordings (Meerlo, de Bruin, Strijkstra, & Daan, 2001). Six hours of SD directly preceding T2, impaired spatial pattern separation performance (Figure 1b). This finding is in line with recent findings in humans that sleep promotes pattern separation (Hanert, Weber, Pedersen, Born, & Bartsch, 2017). Importantly, we observed no differences in total object exploration times when comparing T1 and T2 within experimental conditions or when comparing T1 or T2 exploration times between experimental conditions (p > .05 in all cases, data not shown). The latter analyses indicated that the observed deficits in pattern separation were not due to a total decrease in the explorative activity.
In our previous studies, we showed that the suppression of PDE function prevents memory deficits caused by sleep loss (Havekes, Park, Tudor, et al., 2016; Vecsey et al., 2009). Therefore, as a next step, we assessed whether the pharmacological inhibition of PDE activity makes pattern separation resilient to sleep loss. Importantly, we inhibited PDE activity systemically using drugs that are approved for human use, thereby increasing the translational value of the study (Baillie, Tejeda, & Kelly, 2019; Heckman, Blokland, Bollen, & Prickaerts, 2018; Heckman, Wouters, & Prickaerts, 2015). The following two FDA‐approved PDE inhibitors were used: the PDE4 inhibitor roflumilast (Sigma Aldrich, Zwijndrecht, the Netherlands) and the PDE5 inhibitor vardenafil (kindly donated by BAYER, Wuppertal, Germany). Both PDE inhibitors were previously shown to cross the blood–brain barrier (Akkerman, Blokland, & Prickaerts, 2016; Vanmierlo et al., 2016). We dissolved both drugs daily in the same vehicle solution (98% methyl cellulose tylose solution (0.5%) and 2% Tween80) and administered them in a volume of 2 ml/kg. The drugs were administered i.p. at the start and 3 hr into SD at a dose of 0.03 mg/kg for roflumilast and 0.3 mg/kg for vardenafil. Dosages, volumes, and injection schemes are based on our extensive experience with the current drugs (e.g., Akkerman et al., 2016; Vanmierlo et al., 2016; Vecsey et al., 2009).
First, we focused on the cAMP signaling pathway using the PDE4 inhibitor roflumilast, which improves the hippocampal memory function in animal models as well as young and old human volunteers (Blokland et al., 2019; Van Duinen et al., 2017; Vanmierlo et al., 2016). The systemic delivery of roflumilast during SD successfully prevented the impairment in spatial pattern separation (Figure 2). Importantly, roflumilast exerts its effect at a dose lacking the characteristic emetic side effects observed with classical PDE4 inhibitors (Vanmierlo et al., 2016).
Figure 2.
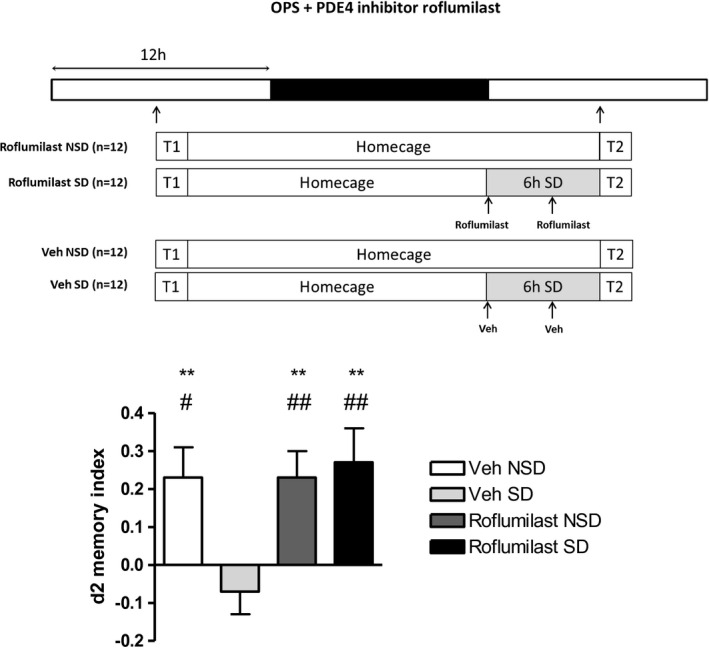
Six hours of SD at the beginning of the light phase directly preceding T2 impairs spatial pattern separation performance (d2 values as mean + SEM) as measured with the OPS test (position 3; 24 h interval). A two‐factor repeated measures ANOVA, using Sleep Deprivation (non‐sleep deprived, sleep deprived) and Treatment (vehicle, roflumilast) as within subject factors, showed a significant interaction effect (F(1,11) = 9.784, p = .010; N = 12). Subsequent post hoc test of simple effects showed that vehicle‐treated SD animals different from all three other experimental conditions (** = p < .01; N = 12; paired samples t test including Bonferroni correction), indicating that roflumilast protects against SD‐induced deficits in spatial pattern separation. # represents a significant difference from zero (# = p < .05, ## = p < .01; N = 12) using independent samples t test. OPS = spatial object pattern separation task; PDE4‐I = phosphodiesterase type 4 inhibitor (roflumilast); Veh = vehicle; SD = sleep deprived; NSD = non‐sleep deprived; T1 = learning trial; T2 = test trial
Because cGMP and cAMP signaling potentially target the same downstream signaling molecules known to be affected by SD (i.e., CREB (Wong et al., 2019), LIMK1‐cofilin (Havekes, Park, Tudor, et al., 2016)), as a next step we targeted cGMP signaling using the PDE5 inhibitor vardenafil. Vardenafil has previously shown to increase cGMP in the hippocampus and improve the memory function in rodents (Akkerman et al., 2016). Treatment with vardenafil also made the pattern separation process resilient to SD (Figure 3).
Figure 3.
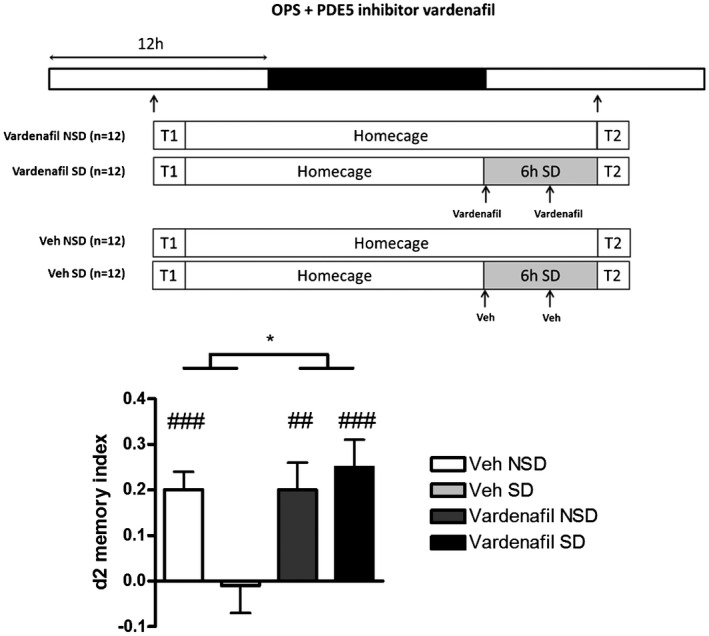
Six hours of SD at the beginning of the light phase directly preceding T2 impairs spatial pattern separation performance (d2 values as mean + SEM) as measured with the OPS test (position 3; 24 h interval). A two‐factor repeated measures ANOVA, using Sleep Deprivation (non‐sleep deprived, sleep deprived) and Treatment (vehicle, vardenafil) as within subject factors, showed no significant interaction effect (F(1,11) = 4.138, p = NS; N = 12), but a main effect for Treatment (F(1,11) = 8.042, p = .016; N = 12), indicating that vardenafil protects against SD‐induced deficits in spatial pattern separation. Additionally, a direct statistical comparison of the “vardenafil SD” and “vehicle SD” groups results in a significant difference (p = .009; paired samples t test). # represents a significant difference from zero (# = p < .05, ## = p < .01) using independent samples t test. NSD = non‐sleep deprived; OPS = spatial object pattern separation task; PDE4‐I = phosphodiesterase type 5 inhibitor (vardenafil); SD = sleep deprived; T1 = learning trial; T2 = test trial; Veh = vehicle.
Together, these results indicate that the inhibition of, respectively, PDE4 or PDE5 signaling with systemic drugs during SD is sufficient to prevent the SD‐induced deficits in spatial pattern separation. A statistical difference between the two PDE inhibitors is that the upregulation of cAMP specifically counters the detrimental effect of SD (interaction effect; Havekes et al., 2014; Havekes, Park, Tudor, et al., 2016; Vecsey et al., 2009), whereas the upregulation of cGMP leads to a general cognition‐enhancing effect (main effect; Akkerman et al., 2016; Argyrousi et al., 2019). However, this difference can be explained by the larger negative discrimination value after SD in the vehicle treatment condition of the roflumilast experiment and may not be a real difference in effect between the PDE inhibitors on the effect of SD on pattern separation. Of note, for the latter, neither any post hoc statistical evidence was found. Altogether, the outcome of these studies makes both inhibitors interesting from a therapeutic perspective as both treatments seem to protect the memory process against the mechanisms by which SD impairs spatial pattern separation.
Previous work has shown that SD decreases CREB phosphorylation in the DG and in parallel upregulates the levels of PDE4A5 in total hippocampal lysates resulting in attenuated levels of cAMP and impaired consolidation of context fear and spatial memories (Havekes, Park, Tolentino, et al., 2016; Vecsey et al., 2009). Decreased levels of hippocampal cAMP negatively impact neuronal connectivity through cAMP‐PKA‐LIMK‐cofilin signaling and attenuating PDE4A5 function prevents these changes including the aforementioned spatial memory deficits (Havekes, Park, Tolentino, et al., 2016; Havekes, Park, Tudor, et al., 2016). Similar changes in neuronal connectivity have been observed in the DG after SD (Raven et al., 2018). In future studies, it will, therefore, be interesting to examine whether the impairments in the OPS are a direct result of increased PDE4A5 function specifically in the DG. The latter would provide functional insight into the molecular underpinnings by which the administration of roflumilast may exert its effect under the conditions of SD.
The role of cGMP and its downstream effectors in spatial pattern separation is currently unknown and to our knowledge, no studies have examined the impact of SD on cGMP signaling in this respect. However, both cyclic nucleotide pathways are known to converge on the CREB and LIMK1‐cofilin pathways (Havekes, Park, Tudor, et al., 2016; Wong et al., 2019; Zulauf et al., 2009), known to play a causal role in the memory and plasticity phenotypes associated with SD. For this reason, it may very well be that treatment with vardenafil prevents deficits in patterns’ separation by acting on the aforementioned signaling pathways.
Finally, it should be noted that both inhibitors did not improve spatial pattern separation in the non‐SD condition. The main reason for the lack of effect on normal (non‐SD) pattern separation could be related to the time of injection as well as the concentration used. In addition, levels of cAMP in non‐SD mice must be higher relative to SD mice. Indeed, in our previous work, we showed that SD reduces basal cAMP levels in the hippocampus (Vecsey et al., 2009). The same may be the cause for cGMP levels although to our knowledge no studies have compared these levels under SD and non‐SD conditions. As such, these higher cAMP levels and potentially higher cGMP levels in non‐SD mice may also contribute to the lack of an effect of PDE4/PDE5 inhibition in non‐SD animals.
In summary, we have demonstrated for the first time that SD negatively impacts spatial pattern separation. As the DG is the main brain area related to spatial pattern separation and specific roles of the cAMP and cGMP pathways in memory processes have been previously described, we tested whether the SD‐induced impairment in spatial pattern separation could be avoided by the application of FDA‐approved PDE inhibitors. Both the PDE4 inhibitor roflumilast and PDE5 inhibitor vardenafil successfully prevented SD‐induced deficits in spatial pattern separation, suggesting that these two FDA‐approved pharmacological inhibitors may be suited to combat the negative impact of SD on cognitive processes.
CONFLICT OF INTEREST
JP has a proprietary interest in the PDE4 inhibitor roflumilast.
Heckman PRA, Roig Kuhn F, Raven F, et al. Phosphodiesterase inhibitors roflumilast and vardenafil prevent sleep deprivation‐induced deficits in spatial pattern separation. Synapse. 2020;74:e22150. 10.1002/syn.22150
Contributor Information
Pim R. A. Heckman, Email: p.r.a.heckman@rug.nl.
Robbert Havekes, Email: r.havekes@rug.nl.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Abel, T. , Havekes, R. , Saletin, J. M. , & Walker, M. P. (2013). Sleep, plasticity and memory from molecules to whole‐brain networks. Current Biology, 23(17), R774–788. 10.1016/j.cub.2013.07.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akkerman, S. , Blokland, A. , & Prickaerts, J. (2016). Possible overlapping time frames of acquisition and consolidation phases in object memory processes: A pharmacological approach. Learning and Memory, 23(1), 29–37. 10.1101/lm.040162.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argyrousi, E. K. , Heckman, P. R. , van Hagen, B. T. , Muysers, H. , van Goethem, N. P. , & Prickaerts, J. (2019). Pro‐cognitive effect of upregulating cyclic guanosine monophosphate signalling during memory acquisition or early consolidation is mediated by increased AMPA receptor trafficking. Journal of Psychopharmacology (Oxford, England), 34(1), 103–114. 10.1177/0269881119885262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baillie, G. S. , Tejeda, G. S. , & Kelly, M. P. (2019). Therapeutic targeting of 3',5'‐cyclic nucleotide phosphodiesterases: Inhibition and beyond. Nature Reviews Drug Discovery, 18(10), 770–796. 10.1038/s41573-019-0033-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blokland, A. , Van Duinen, M. A. , Sambeth, A. , Heckman, P. R. A. , Tsai, M. , Lahu, G. , … Prickaerts, J. (2019). Acute treatment with the PDE4 inhibitor roflumilast improves verbal word memory in healthy old individuals: A double‐blind placebo‐controlled study. Neurobiology of Aging, 77, 37–43. 10.1016/j.neurobiolaging.2019.01.014 [DOI] [PubMed] [Google Scholar]
- Dillon, S. E. , Tsivos, D. , Knight, M. , McCann, B. , Pennington, C. , Shiel, A. I. , … Coulthard, E. J. (2017). The impact of ageing reveals distinct roles for human dentate gyrus and CA3 in pattern separation and object recognition memory. Scientific Reports, 7(1), 14069. 10.1038/s41598-017-13853-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanert, A. , Weber, F. D. , Pedersen, A. , Born, J. , & Bartsch, T. (2017). Sleep in humans stabilizes pattern separation performance. Journal of Neuroscience, 37(50), 12238–12246. 10.1523/jneurosci.1189-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havekes, R. , & Abel, T. (2017). The tired hippocampus: The molecular impact of sleep deprivation on hippocampal function. Current Opinion in Neurobiology, 44, 13–19. 10.1016/j.conb.2017.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havekes, R. , Bruinenberg, V. M. , Tudor, J. C. , Ferri, S. L. , Baumann, A. , Meerlo, P. , & Abel, T. (2014). Transiently increasing cAMP levels selectively in hippocampal excitatory neurons during sleep deprivation prevents memory deficits caused by sleep loss. Journal of Neuroscience, 34(47), 15715–15721. 10.1523/jneurosci.2403-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havekes, R. , Meerlo, P. , & Abel, T. (2015). Animal studies on the role of sleep in memory: From behavioral performance to molecular mechanisms. Current Topics in Behavioral Neurosciences, 25, 183–206. 10.1007/7854_2015_369 [DOI] [PubMed] [Google Scholar]
- Havekes, R. , Park, A. J. , Tolentino, R. E. , Bruinenberg, V. M. , Tudor, J. C. , Lee, Y. , … Abel, T. (2016). Compartmentalized PDE4A5 signaling impairs hippocampal synaptic plasticity and long‐term memory. Journal of Neuroscience, 36(34), 8936–8946. 10.1523/jneurosci.0248-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havekes, R. , Park, A. J. , Tudor, J. C. , Luczak, V. G. , Hansen, R. T. , Ferri, S. L. , … Abel, T. (2016). Sleep deprivation causes memory deficits by negatively impacting neuronal connectivity in hippocampal area CA1. Elife, 5, e13424. 10.7554/eLife.13424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckman, P. R. A. , Blokland, A. , Bollen, E. P. P. , & Prickaerts, J. (2018). Phosphodiesterase inhibition and modulation of corticostriatal and hippocampal circuits: Clinical overview and translational considerations. Neuroscience and Biobehavioral Reviews, 87, 233–254. 10.1016/j.neubiorev.2018.02.007 [DOI] [PubMed] [Google Scholar]
- Heckman, P. R. , Wouters, C. , & Prickaerts, J. (2015). Phosphodiesterase inhibitors as a target for cognition enhancement in aging and Alzheimer's disease: A translational overview. Current Pharmaceutical Design, 21(3), 317–331. [DOI] [PubMed] [Google Scholar]
- Kheirbek, M. A. , Klemenhagen, K. C. , Sahay, A. , & Hen, R. (2012). Neurogenesis and generalization: A new approach to stratify and treat anxiety disorders. Nature Neuroscience, 15(12), 1613–1620. 10.1038/nn.3262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreutzmann, J. C. , Havekes, R. , Abel, T. , & Meerlo, P. (2015). Sleep deprivation and hippocampal vulnerability: Changes in neuronal plasticity, neurogenesis and cognitive function. Neuroscience, 309, 173–190. 10.1016/j.neuroscience.2015.04.053 [DOI] [PubMed] [Google Scholar]
- McGaugh, J. L. (2000). Memory–A century of consolidation. Science, 287(5451), 248–251. [DOI] [PubMed] [Google Scholar]
- Meerlo, P. , de Bruin, E. A. , Strijkstra, A. M. , & Daan, S. (2001). A social conflict increases EEG slow‐wave activity during subsequent sleep. Physiology and Behavior, 73(3), 331–335. 10.1016/s0031-9384(01)00451-6 [DOI] [PubMed] [Google Scholar]
- Meerlo, P. , Mistlberger, R. E. , Jacobs, B. L. , Heller, H. C. , & McGinty, D. (2009). New neurons in the adult brain: The role of sleep and consequences of sleep loss. Sleep Medicine Reviews, 13(3), 187–194. 10.1016/j.smrv.2008.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raven, F. , Meerlo, P. , Van der Zee, E. A. , Abel, T. , & Havekes, R. (2018). A brief period of sleep deprivation causes spine loss in the dentate gyrus of mice. Neurobiology of Learning and Memory, 160, 83–90. 10.1016/j.nlm.2018.03.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Duinen, M. A. , Sambeth, A. , Heckman, P. , Smit, S. , Tsai, M. , Lahu, G. , … Prickaerts, J. (2017). Acute administration of roflumilast enhances immediate recall of verbal word memory in healthy young adults. Neuropharmacology, 131, 31–38. 10.1016/j.neuropharm.2017.12.019 [DOI] [PubMed] [Google Scholar]
- Van Erum, J. , Van Dam, D. , & De Deyn, P. P. (2019). Alzheimer's disease: Neurotransmitters of the sleep‐wake cycle. Neuroscience and Biobehavioral Reviews, 105, 72–80. 10.1016/j.neubiorev.2019.07.019 [DOI] [PubMed] [Google Scholar]
- van Goethem, N. P. , Schreiber, R. , Newman‐Tancredi, A. , Varney, M. , & Prickaerts, J. (2015). Divergent effects of the “biased” 5‐HT1 A receptor agonists F15599 and F13714 in a novel object pattern separation task. British Journal of Pharmacology, 172(10), 2532–2543. 10.1111/bph.13071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Goethem, N. P. , van Hagen, B. T. J. , & Prickaerts, J. (2018). Assessing spatial pattern separation in rodents using the object pattern separation task. Nature Protocols, 13(8), 1763–1792. 10.1038/s41596-018-0013-x [DOI] [PubMed] [Google Scholar]
- van Os, J. , & Kapur, S. (2009). Schizophrenia. Lancet, 374(9690), 635–645. 10.1016/s0140-6736(09)60995-8 [DOI] [PubMed] [Google Scholar]
- Vanmierlo, T. , Creemers, P. , Akkerman, S. , van Duinen, M. , Sambeth, A. , De Vry, J. , … Prickaerts, J. (2016). The PDE4 inhibitor roflumilast improves memory in rodents at non‐emetic doses. Behavioural Brain Research, 303, 26–33. 10.1016/j.bbr.2016.01.031 [DOI] [PubMed] [Google Scholar]
- Vecsey, C. G. , Baillie, G. S. , Jaganath, D. , Havekes, R. , Daniels, A. , Wimmer, M. , … Abel, T. (2009). Sleep deprivation impairs cAMP signalling in the hippocampus. Nature, 461(7267), 1122–1125. 10.1038/nature08488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong, L. W. , Tann, J. Y. , Ibanez, C. F. , & Sajikumar, S. (2019). The p75 neurotrophin receptor is an essential mediator of impairments in hippocampal‐dependent associative plasticity and memory induced by sleep deprivation. Journal of Neuroscience, 39(28), 5452–5465. 10.1523/jneurosci.2876-18.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassa, M. A. , & Stark, C. E. (2011). Pattern separation in the hippocampus. Trends in Neurosciences, 34(10), 515–525. 10.1016/j.tins.2011.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zulauf, L. , Coste, O. , Marian, C. , Moser, C. , Brenneis, C. , & Niederberger, E. (2009). Cofilin phosphorylation is involved in nitric oxide/cGMP‐mediated nociception. Biochemical and Biophysical Research Communications, 390(4), 1408–1413. 10.1016/j.bbrc.2009.10.166 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


