FIGURE 2.
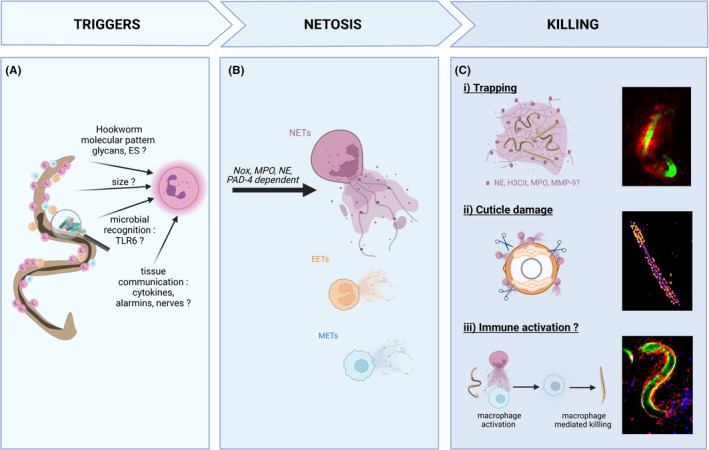
Neutrophil extracellular traps kill larvae via a variety of mechanisms. Neutrophil extracellular traps (NETs) are induced in response to hookworm and related helminth larvae. (A) The mechanism of NETs induction is not yet characterized. The current hypothesis include parasite‐specific products such as glycans and excretory/secretory (ES) products, the multicellular size of larvae, recognition of microbiome/soil‐derived microbial signatures via toll‐like receptors (TLR), or indirect activation from as‐yet‐unknown immune or non‐immune cells. (B) Following hookworm detection, NETosis induction requires NADPH oxidase (Nox), myeloperoxidase (MPO), neutrophil elastase (NE), and peptidylarginine deiminase 4 (PAD4). While neutrophils have been the primary study of hookworm‐induced NETosis, emerging evidence suggests other cells may form extracellular traps such as eosinophils (EETs) and monocytes/macrophages (METs). (C) In the absence of immuno‐evasion, NETs can participate in larval killing by direct or indirect mechanisms not mutually exclusive: (i) Larvae are mechanically trapped by NETs. L3 are potentially exposed to a high concentration of “decorating” enzymes (NE, citrullinated histone H3 (H3Cit), MPO, and matrix metalloprotease 9 [MMP‐9]) or killed by other immune cells recruited to the traps and (ii) the cuticle of larvae is damaged by neutrophil enzymes such as NE, causing increased permeability to sytox green. (iii) NETs directly activate other immune cells, such as macrophage to potentiate their larvicidal activity. Immunofluorescence microscopy of Necator brasiliensis L3: (i) intravital imaging of larvae in the skin (CFSE stained, green) trapped by NETs stained with the DNA binding dye sytox blue (red). (ii) Larvae killed by NETs in vitro and stained with sytox green for 3 h. The damaged cuticle lets the otherwise impermeant dye through to stain the internal structures of the worm. Fluorescence intensity represented with the fire LUT in Fiji. (iii) Intravital imaging of neutrophils (Ly6G‐PE red) adhered to larvae (CFSE‐stained, green) surrounded by monocytes (Ly6C‐BV421, blue) 6 h post intradermal inoculation. For more details about the methodology, please see. 20 The figure has been made using Biorender
