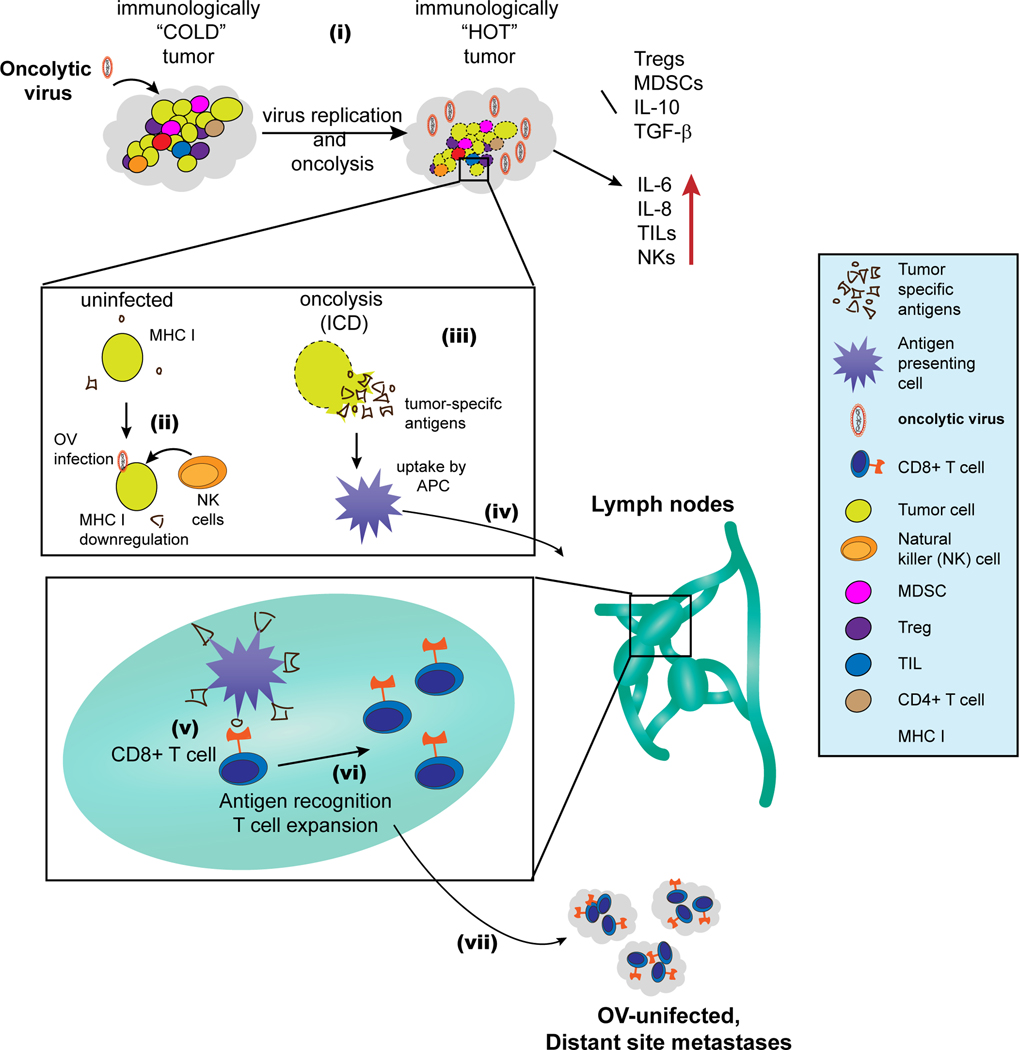
Figure 1. Modulation of the tumor microenvironment by OVs and elicitation of anti-tumor immunity.
(i) OVs convert immunologically “COLD” tumors to immunologically “HOT” tumors: tumors generally have high concentration of immunesuppressive cells and cytokines, which make them immunologically less responsive i.e. immunologically “COLD”. OVs induce inflammation and inhibit immunosuppressive cells (Tregs and MDSCs) and cytokines (IL-10 and TGF-β). Conversely, OVs also increase proinflammatory cytokines (IL-6 and IL-8) and foster tumor infiltration by NK cells and other TILs. This complex modulation of the TME by OVs converts the tumor to inflamed, immunologically “HOT”. (ii) OV infection increases NK cell-mediated killing of tumor cells: tumor cells tend to reduce their MHC I levels in response to virus infection. Reduction in MHC I allows recognition and killing of virus infected cancer cells by NK cells. (iii) Oncolysis by OVs causes the release of tumor-associated/specific antigens, and OVs also induce immunogenic cell death (ICD). (iv) Antigen-loaded APCs migrate to the lymph node, where (v) they cross-present tumor antigens to CD8+ T cells. (vi) Following activation, the tumor-specific CD8+ T cells undergo expansion. (vii) Finally, the tumor-specific T cells move to both OV injected and uninjected tumors (distant metastases) where they can exert anti-tumor effect.