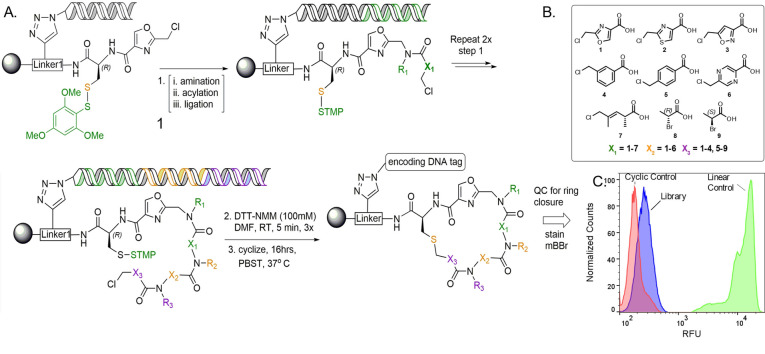
Figure 2.
General library design. A) The library was synthesized by solid‐phase split‐and‐pool synthesis on 10 μm resin possessing a linker (Linker‐1) modified with HDNA, Cys(STmp), and an oxazole (Compound 1). The linear precursors were formed after 3 rounds of amination/acylation/enzymatic ligation using the backbone units shown (B). The STmp group was then removed to allow thioether formation. C) After macrocyclization, aliquots (about 10 000 beads) were stained with a thiol‐reactive fluorescent dye (mBBr) to confirm that the vast majority of the library completed cyclization. Control compounds are shown in Figure S5.