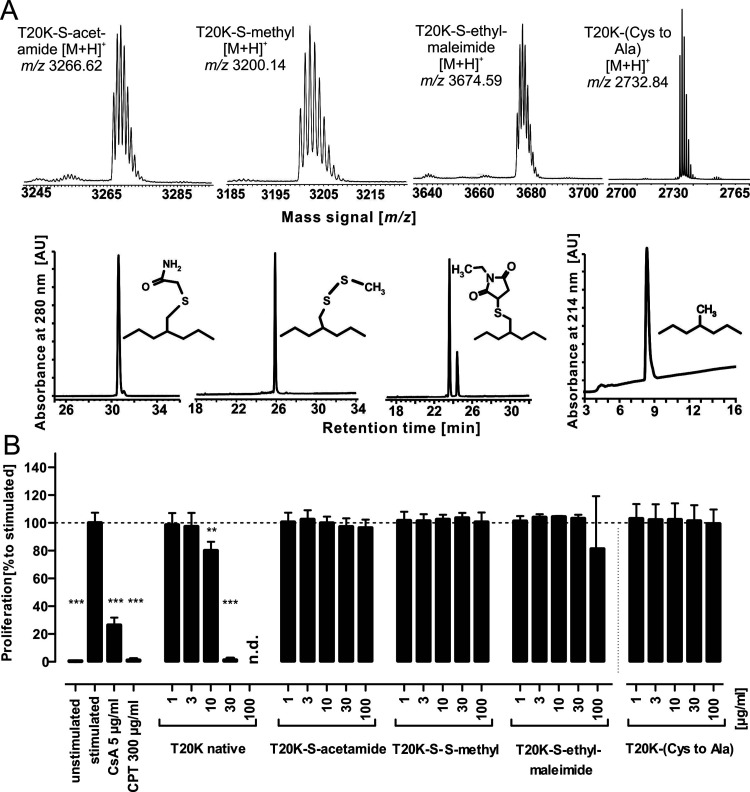
Figure 2.
Cystine knot truncated variants. (A) T20K was reduced with dithiothreitol, and free-reactive sulfhydryl moieties were derivatized with site-specific reagents iodoacetamide, methyl methanethiosulfonate, and N-ethyl-maleimide, providing the S-acetamide, the -S-S-methyl, and the S-ethyl-maleimide derivative, respectively. Following HPLC purification, the identities of any derivative were confirmed by mass spectrometry. Additionally, the probe T20K-(Cys-to-Ala), where all six cysteines were replaced with the isostere alanine, was prepared to rule out steric hindrance of sulfhydryl derivatization. (B) All three cysteine-derivatized peptides as well as the variant with isosteres showed a total loss of antiproliferative activity in the tested concentration range of 1–100 μg/mL peptide. All data represent the mean ± standard deviation of three biological replicates, expressed relative to the stimulated control (≙100%), The data for the T20K-(Cys-to-Ala) were normalized to the stimulated control shown in Figure S1. CsA and CPT are positive controls. Asterisks (**p < 0.01, ***p < 0.001) indicate significant differences compared to stimulated control, and (n.d.) indicates data not detected.