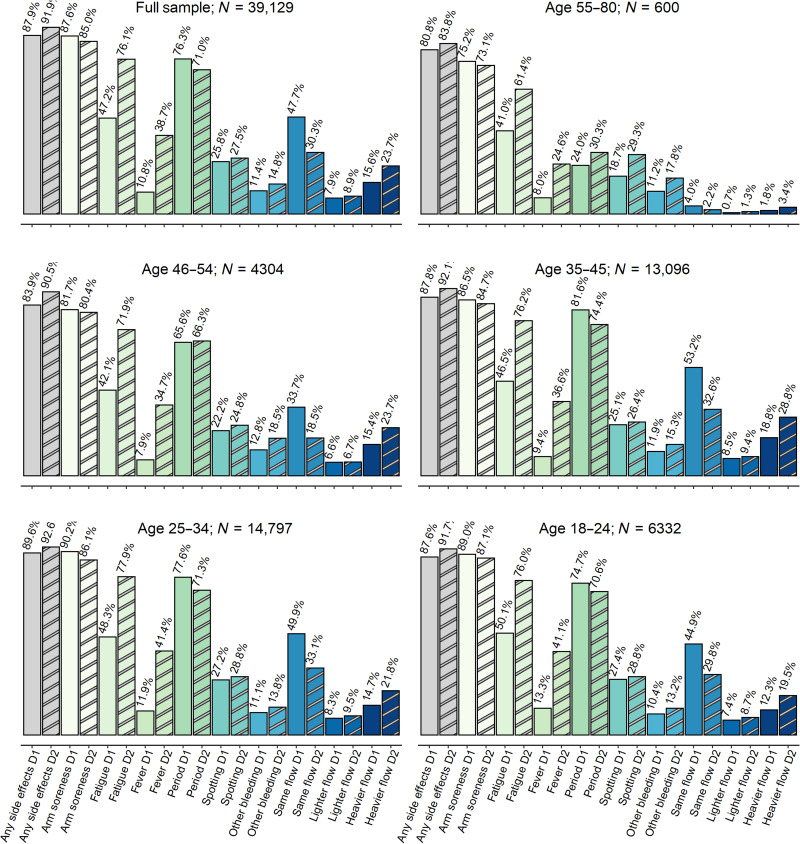
Fig. 2. Descriptive statistics of the full sample (dose 1 displayed in solid bars and dose 2 displayed in striped bars).
The most salient vaccine and menstrual side effects pertaining to the analysis are presented here. The sample sizes of dose 2 variables decrease because of those who received the one-dose Johnson & Johnson vaccine. The respective samples become as follows: full, N = 35,660; age 18 to 24, N = 5698; age 25 to 34, N = 13,537; age 35 to 45, N = 11,970; age 46 to 54, N = 3898; age 55 to 80, N = 557.