Abstract
The body and brain are in constant two‐way communication. Driving this communication is a region in the lower brainstem: the dorsal vagal complex. Within the dorsal vagal complex, the caudal nucleus of the solitary tract (cNTS) is a major first stop for incoming information from the body to the brain carried by the vagus nerve. The anatomy of this region makes it ideally positioned to respond to signals of change in both emotional and bodily states. In turn, the cNTS controls the activity of regions throughout the brain that are involved in the control of both behaviour and physiology. This review is intended to help anyone with an interest in the cNTS. First, I provide an overview of the architecture of the cNTS and outline the wide range of neurotransmitters expressed in subsets of neurons in the cNTS. Next, in detail, I discuss the known inputs and outputs of the cNTS and briefly highlight what is known regarding the neurochemical makeup and function of those connections. Then, I discuss one group of cNTS neurons: glucagon‐like peptide‐1 (GLP‐1)‐expressing neurons. GLP‐1 neurons serve as a good example of a group of cNTS neurons, which receive input from varied sources and have the ability to modulate both behaviour and physiology. Finally, I consider what we might learn about other cNTS neurons from our study of GLP‐1 neurons and why it is important to remember that the manipulation of molecularly defined subsets of cNTS neurons is likely to affect physiology and behaviours beyond those monitored in individual experiments.
Keywords: afferent, anatomy, efferent, glucagon‐like peptide‐1, neuropeptides, nucleus of the solitary tract
1. INTRODUCTION
Our emotional and physical well‐being is carefully monitored by the brain through multimodal pathways. Hormonal input is carried to the brain from the body via the blood, whereas the spinal cord and cranial nerves, including the vagus nerve, carry electrical signals from the periphery to the brain. 1 , 2 The afferent (sensory) vagus terminates, among other brainstem nuclei, in the dorsal vagal complex in the lower brainstem. The dorsal vagal complex comprises the nucleus of the solitary tract (NTS), the area postrema (AP) and the dorsal motor nucleus of the vagus (DMV) (Figure 1A,B), with the bulk of the vagal input terminating on second‐order neurons in the caudal part of the NTS (cNTS). 2 This makes the cNTS anatomically unusual: it receives direct sensory input from the afferent vagus and spinal cord, as well as descending inputs from higher brain regions. This configuration places the cNTS in an ideal position to integrate cognitive information with interoceptive input. Indeed, the cNTS is activated following both interoceptive and psychogenic stimuli. 3 In turn, the cNTS modulates multiple processes from autonomic outflow to motivated behaviour. 1 , 4 Unfortunately, much of the anatomical organization uncovered in the last century is often forgotten in contemporary neuroscience reports, perhaps as a result of the lack of easily accessible, recent overviews. This review is intended to provide exactly that: an overview of the efferent and afferent connections of the NTS, as well as the current state of knowledge regarding the neuropeptidergic cell types residing within the cNTS.
FIGURE 1.
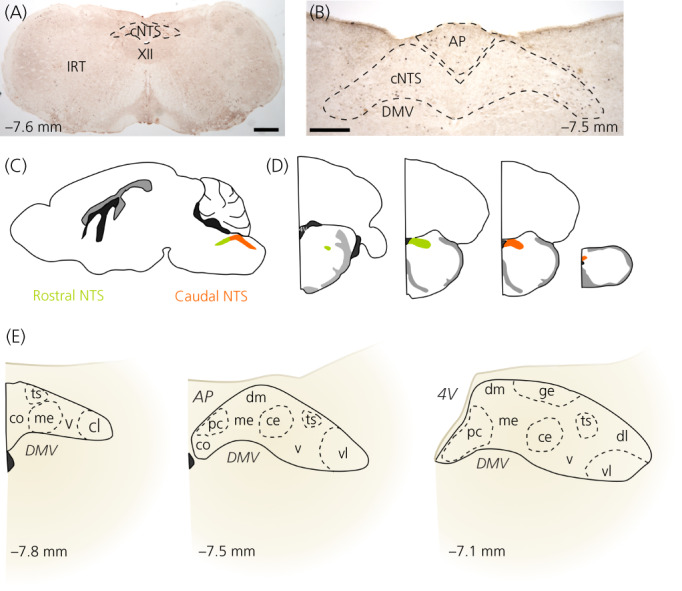
Anatomical organization of the caudal nucleus of the solitary tract (cNTS). (A) Coronal brainstem section containing the cNTS. The hypoglossal nucleus (XII) and the intermediate reticular nucleus (IRT) are indicated as landmarks. Labelled for cFOS (black product) and glucagon‐like peptide‐1 (GLP‐1) (brown product) using immunohistochemistry. Scale bar = 400 μm. (B) Higher magnification of the dorsal vagal complex containing the area postrema (AP), cNTS and dorsal motor nucleus of the vagus (DMV). Labelled for cFOS (black product) and GLP‐1 (brown product) using immunohistochemistry. Scale bar = 200 μm. (C) Schematic of mouse brain with rostral and caudal NTS indicated in green and orange. (D) Schematics of coronal sections through different rostrocaudal levels of the NTS with the rostral part indicated in green and the caudal part indicated in orange. (E) Schematics of cNTS at different rostrocaudal levels (indicated in millimetre from Bregma) with the subnuclei indicated. 4V, fourth ventricle; Ts, solitary tract; co, commissural nucleus; me, medial nucleus; v, ventral nucleus; cl, caudolateral nucleus; pc, parvocellular nucleus; dm, dorsomedial nucleus; ce, central nucleus; vl, ventrolateral medulla; dl, dorsolateral medulla
First, I briefly describe the architecture of the cNTS and its resident cell types. Then, I review the anatomical configuration of the inputs and outputs of the cNTS. Finally, as perhaps the best studied peptidergic population of cNTS neurons, glucagon‐like peptide‐1 (GLP‐1)‐expressing neurons will be used as an example of second‐order neurons, which receive substantial vagal sensory input, as well as input from both forebrain and hindbrain regions. This review will not cover the function of the cNTS in detail, and readers are referred to excellent available reviews on the subject. 1 , 3 , 4 , 5 , 6
An important note on species is warranted: this review covers only preclinical data, most of which was collected in rodents. Indeed, most of the anatomical data that will be discussed were collected in rat. A small number of tracing and cytoarchitecture studies have been conducted in rabbit, 7 hamster 8 and cat, 9 and, more recently, presumably as a result of the increased popularity of the mouse as an experimental model, reports of the anatomy of the mouse NTS have been added to the literature. 10 , 11 , 12 For clarity and to emphasize the idea that not all species can be reasonably assumed to be anatomically and functionally identical, I will indicate the species that the data were collected from.
2. NTS ARCHITECTURE
In rodents, the NTS is traditionally, if somewhat arbitrarily, divided into two parts, sometimes three, 13 based on their relative rostrocaudal location 10 , 14 : The rostral or gustatory part of the NTS buds dorsolaterally from the spinal trigeminal nucleus to the level of the closure of the fourth ventricle and formation of the AP. It is termed gustatory, because this part of the NTS is the first relay in the central taste neuraxis. The caudal or visceral NTS, which receives vagal afferent input originating in the viscera, extends from the opening of the fourth ventricle to the junction between the spinal cord and the lower brainstem (Figure 1C). In coronal sections, the NTS is oval in appearance at more rostral levels (Figure 1D) but takes a triangular shape at the level of the AP, with the DMV situated at its ventral border. At its rostral extreme, the NTS is at its most lateral and gradually moves more medial until it finally surrounds the midline at the very caudal end of the nucleus (Figure 1D).
In rodents, the NTS can be subdivided into a number of subnuclei based on the location, size, shape, density, and staining intensity of neuronal cell bodies following Nissl, silver or Golgi staining. Following this approach, Ganchrow et al. 10 thoroughly mapped the cytoarchitecture of the mouse NTS and compared this to previous reports in hamster and rat, 10 which were largely similar. Because Ganchrow et al. 10 provides such an excellent and exhaustive description of the subnuclear organization of the rodent NTS, this particular aspect of NTS anatomy will not be described in detail here. However, for convenience Figure 1E presents an overview of the subnuclear division of the cNTS.
3. CELL TYPES OF THE CNTS
The cNTS is cellularly heterogeneous with a multitude of neuropeptides (Figure 2), small‐molecule neurotransmitters and receptors expressed in distinct or overlapping neuronal populations (Table 1). Not all have been investigated in detail beyond demonstrating their expression in the cNTS and only few have been selectively targeted to study their physiological roles (Figure 2, Table 1). In the last decade, advances in chemo‐ and optogenetic manipulation have made it possible to selectively activate or inhibit cells in an anatomically and genetically defined manner. These advances will not be discussed in detail here, but are highlighted with appropriate references in Figure 2 and Table 1. It is important to note that not all of the listed molecules have been confirmed to be expressed exclusively by neurons. Indeed, astrocytes express both leptin receptors 81 and GLP‐1 receptors. 82
FIGURE 2.
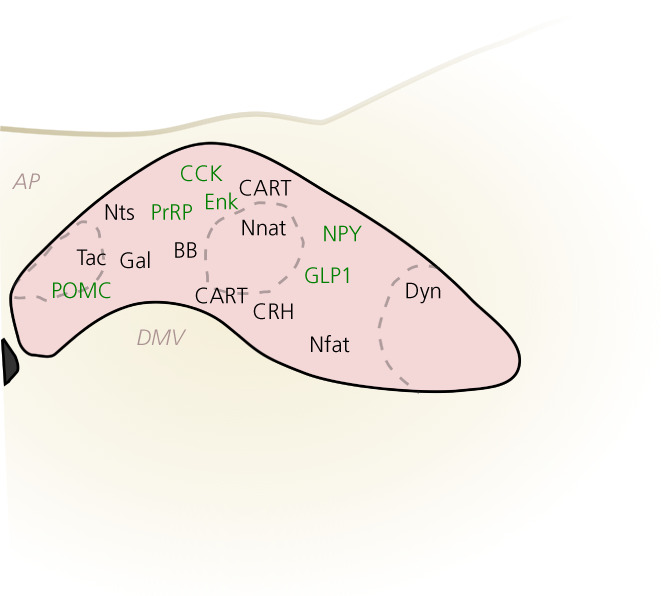
Known peptidergic cell types of the caudal nucleus of the solitary tract (cNTS). Cell types with approximate locations based on published studies referenced in Table 1. Highlighted in green are cell types that have been manipulated chemo‐ or optogenetically to investigate their function as indicated in Table 1. There is conflicting evidence on the location of cocaine‐ and amphetamine‐regulated transcript (CART) neurons, possibly as a result of species differences. For details, see Table 1. Abbreviations are indicated in Table 2
TABLE 1.
Expression of neuropeptides and selected small‐molecular neurotransmitters, intracellular proteins, and receptors in the caudal nucleus of the solitary tract (cNTS)
| Neuronal population | Detected in species | Response to cell type‐specific manipulations | References |
|---|---|---|---|
| Neuropeptides | |||
| Bombesin‐like peptides (BB) | Mouse, rat | – | 15, 16 |
| Cocaine‐ and amphetamine‐regulated transcript (CART) | Mouse, rat | – | 17, 18, 19, 20 |
| Cholecystokinin‐8 (CCK) | Mouse, rat |
Chemogenetic activation (whole population): food intake↓; conditioned place avoidance; condition taste avoidance Optogenetic activation (fibres in parabrachial nucleus [PBN]): food intake↓; real‐time place avoidance Optogenetic activation (fibres in paraventricular nucleus [PVN]): food intake↓; real‐time place preference |
19, 21, 22, 23, 24, 25, 26, 27 |
| Corticotropin‐releasing hormone (CRH) | Mouse, rat | – | 26, 28, 29 |
| Dynorphin (Dyn) | Rat | – | 27, 30 |
| Enkephalin (Enk) | Rat, mouse | Chemogenetic activation: novel flavour preference↑ | 27, 30, 31 |
| Galanin (Gal) | Rat, mouse | – | 26, 32, 33, 34 |
| Glucagon‐like peptide‐1 (GLP1) | Mouse, rat |
Optogenetic activation (whole population and fibres in PVN): food intake↓; Chemogenetic activation: food intake↓; heart rate↑; locomotion↓; glucose production↓; drug reward↓. Chemogenetic inhibition: fast‐refeed↑; stress‐induced hypophagia↓ |
35, 36, 37, 38, 39, 40, 41, 42, 43, 44 |
| Neuronatin (Nnat) | Rat | – | 45 |
| Neuropeptide Y (NPY) | Mouse, rat | Chemogenetic activation: food intake↑ | 19, 27, 46, 47 |
| Neurotensin (Nts) | Mouse, rat | – | 31, 48, 49 |
| Nesfatin‐1 (Nfat) | Mouse, rat | – | 19, 50, 51, 52, 53 |
| Proopiomelanocortin (POMC) | Mouse, rat |
Optogenetic activation: heart rate↓; breathing↓; Chemogenetic activation: nociception↓; food intake↓; Ablation: food intake↑ |
17, 54, 55, 56, 57 |
| Prolactin‐releasing peptide (PrRP) | Mouse, rat |
Chemogenetic activation: food intake↓ Chemogenetic inhibition: fast‐refeed↑ Ablation: diet‐induced obesity↑ |
19, 58, 59, 60 |
| Tachykinin/substance P (Tac) | Rat | – | 27, 61 |
| Small molecules | |||
| GABA | Mouse, rat | Chemogenetic activation: blood glucose↑ | 62, 63, 64, 65 |
| Glutamate | Mouse, rat | Optogenetic activation: renal and phrenic sympathetic nerve activity↑ | 66 |
| Noradrenaline (NA) | Mouse, rat |
Chemogenetic activation (NET‐Cre; DBH‐Cre): food intake↓ Optogenetic activation (DBH‐cre; fibres in PBN): food intake↓ Optogenetic activation (TH‐cre; fibres in Arc): food intake↑ |
22, 47, 67 |
| Intracellular proteins | |||
| Brain‐derived neurotrophic factor (BDNF) | Mouse, rat | – | 19, 68, 69 |
| 11β‐hydroxysteroid dehydrogenase 2 (HSD2) | Mouse |
Ablation: sodium appetite↓ Chemogenetic activation: sodium appetite↑ Optogenetic activation (fibres in bed nucleus of the stria terminalis): sodium appetite↑ |
70 |
| Phox2B | Mouse, rat |
Chemogenetic activation: breathing↑; food intake↓ Ablation: breathing↓ |
71, 72 |
| Neuronal nitric oxide synthase (nNOS) | Rat | – | 73 |
| Receptors and transporters | |||
| Angiotensin‐II receptor (AT2R) | Mouse | Optogenetic activation: systemic blood pressure↑; heart rate↑ | 74 |
| Glucose transporter 2 (GLUT2) | Mouse | Optogenetic activation: vagal efferent activity↑; blood glucagon↑ | 75 |
| GLP1 receptor (GLP1R) | Rat, mouse | – | 42, 76, 77 |
| Leptin receptor (LEPR) | Mouse, rat |
Optogenetic and chemogenetic activation (whole population): breathing↑; food intake↓ Chemogenetic activation (PBN‐projecting): breathing↑ |
32, 78, 79 |
| Calcitonin receptor (CALCR) | Mouse |
Chemogenetic activation: food intake↓ Optogenetic activation (fibres in PBN): food intake↓ Chemogenetic inhibition: food intake↑ Ablation: food intake↑ |
23 |
| 5‐hydroxytryptamine 2C receptor (5‐HT2CR) | Mouse, rat |
Chemogenetic activation: food intake↓ |
80 |
Note: Only those with anatomical evidence for expression within neuronal populations resident in the NTS are included. As such, evidence based on physiological or behavioural responses to microinjection of agonists or antagonists into the cNTS has not been included. Also indicated are effects of opto‐ or chemogenetic manipulations of the cellular activity of the particular subpopulation. The list of receptors and transporters is not exhaustive, but highlights a few well‐studied examples.
Abbreviations: DBH, Dopamine β‐hydroxylase; NET, Norepinephrine transporter; TH, Tyrosine hydroxylase.
TABLE 2.
Abbreviations
| IX | 9th cranial nerve, glossopharyngeal nerve |
|---|---|
| X | 10th cranial nerve, vagus nerve |
| AAV | Adeno‐associated virus |
| AGRP | Agouti‐related peptide |
| AP | Area postrema |
| Arc | Arcuate nucleus |
| Bar | Barrington's nucleus |
| BB | Bombesin‐like peptides |
| BST | Bed nucleus of the stria terminalis |
| CART | Cocaine‐ and amphetamine‐regulated transcript |
| CCK | Cholecystokinin‐8 |
| CeA | Central amygdala |
| CRH | Corticotropin‐releasing hormone |
| CTb | Choleratoxin subunit b |
| DH | Dorsal horn of the spinal cord |
| DMH | Dorsomedial hypothalamus |
| DMV | Dorsal motor nucleus of the vagus |
| DR | Dorsal raphe |
| Dyn | Dynorphin |
| Enk | Enkephalin |
| Gal | Galanin |
| Gi | Gigantocellular nucleus |
| GLP1 | Glucagon‐like peptide‐1 |
| IC | Insular cortex |
| IL | Infralimbic cortex |
| IML | Intermediolateral column in the spinal cord |
| IRT | Intermediate reticular formation |
| IX | Glossopharyngeal nerve |
| KF | Kölliker‐Fuse nucleus |
| LC | Locus coeruleus |
| LH | Lateral hypothalamus |
| MS | Medial septum |
| NAc | Nucleus Accumbens |
| Nfat | Nesfatin‐1 |
| Nnat | Neuronatin |
| NPY | Neuropeptide Y |
| Nts | Neurotensin |
| NTS | Nucleus of the solitary tract |
| cNTS | Caudal nucleus of the solitary tract |
| OT | Oxytocin |
| OVLT | Vascular organ of lamina terminalis |
| PAG | Periaqueductal grey |
| PBN | Parabrachial nucleus |
| POMC | Proopiomelanocortin |
| PPY | Parapyramidal region |
| PrL | Prelimbic cortex |
| PrRP | Prolactin‐releasing peptide |
| PSTh | Parasubthalamic nucleus |
| PVN | Paraventricular nucleus of the hypothalamus |
| PVT | Paraventricular nucleus of the thalamus |
| RLi | Linear raphe nucleus |
| RMg | Raphe magnus |
| ROb | Raphe obscurus |
| RPa | Raphe pallidus |
| SFO | Subfornical organ |
| SI | Substantia innominata |
| Sp5 | Spinal trigeminal nucleus |
| Tac | Tachykinin/substance P |
| VH | Ventral horn of the spinal cord |
| VLM | Ventrolateral medulla |
| VMH | Ventromedial hypothalamus |
| VTA | Ventral tegmental area |
| ZI | Zona incerta |
3.1. NTS glia in the modulation of information flow in the NTS
In addition to neurons, astrocytes contribute heavily to the function of the cNTS. 5 Based on immunolabelling for glial‐fibrillary protein (GFAP), astrocytes appear to be more densely packed in the rat NTS 82 , 83 than in the mouse NTS, 76 , 84 although, to this author's knowledge, a direct, quantitative comparison has not been made. Of note, in both species, the densest expression of GFAP is found in the border region between the AP and the cNTS, 83 , 84 , 85 where astrocytes may regulate transport of molecules across the border and thus modulate the flow of information from the blood into the NTS. 81 , 85 In addition to forming a selectively permeable diffusion barrier between the AP and the NTS, astrocytes in the rat NTS form part of tripartite synapses, specialized synaptic arrangements consisting of a synaptic cleft containing the pre‐ and postsynaptic terminals covered by astrocytic processes. 86 Interestingly, NTS astrocytes are activated in response to vagal stimulation in rats and NTS gliotransmission modulates the synaptic transmission of second‐order NTS neurons in rats. 87
In addition to astrocytes, microglia and oligodendrocytes express neurotransmitter receptors and microglia in the NTS are altered in response to varied stimuli, including removal of vagal input 83 (rat), obesity 88 (rat), and hypoxia 89 (mouse). A detailed discussion of NTS glial function is beyond the scope of this review, and readers are referred to a recent comprehensive review on the subject. 5 , 90 , 91
4. AFFERENT CONNECTIONS OF THE CNTS
Input to the cNTS arises from widespread regions in the brain, as well as peripheral sites (Figure 3), comprising an anatomical organization that is reminiscent of the significant variety of physiological and psychogenic stimuli, which modulate the activity of the NTS. 92 These inputs have been reported predominantly in rats, although studies using mice, rabbits and cats are also included here. Studies mapping the monosynaptic input to the NTS take one of two forms: (1) injection of an anterograde tracer (typically phaseolus vulgaris leucoagglutinin [PHA‐L], 10,000 MW biotin dextran amine [BDA], or adeno‐associated virus [AAV]) from a hypothesized source of input to the NTS and subsequent validation of the presence of labelled axons in the NTS or (2) injection of a retrograde tracer (typically wheatgerm agglutinin‐horseradish peroxidase [WGA‐HRP], choleratoxin subunit B [CTb], fluorogold, or a retrograde AAV) into the NTS and subsequent mapping of retrogradely labelled brain regions. In addition, a few studies have mapped inputs to molecular defined subpopulations of cNTS neurons using cell‐type specific monosynaptic and polysynaptic retrograde tracing. 12 , 93
FIGURE 3.
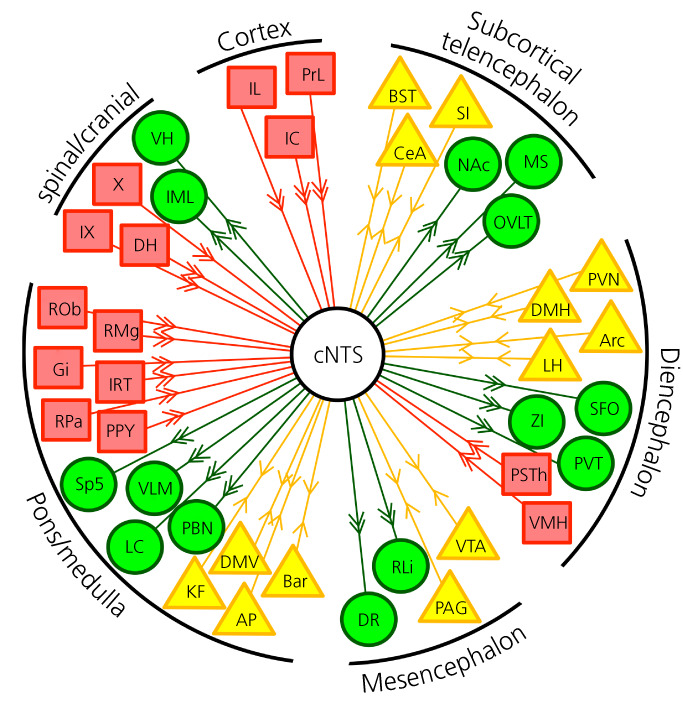
Efferent and afferent inputs to the caudal nucleus of the solitary tract (cNTS). Direct inputs to the cNTS are indicated in red boxes. Projection targets are indicated in green circles. Areas with bidirectional connections are in yellow triangles. For abbreviations, see Table 2
4.1. Sensory inputs to the cNTS
The cNTS is directly sensitive to blood‐borne signals, including changes in glucose, 94 leptin 46 and angiotensin II. 95 In addition, visceral sensory information is transmitted via the afferent vagus and glossopharyngeal nerves to the cNTS where glutamatergic terminals synapse onto second‐order neurons. 1 , 96 , 97 Peripheral chemo‐ and baroreceptors sense changes in blood pressure, as well as the pH, temperature and composition of the arterial blood. 6 This information is relayed by the afferent glossopharyngeal and vagus nerves to the NTS. 6 In addition to the continuous monitoring of cardiovascular and pulmonary function, the cNTS receives information from the abdominal viscera via the afferent vagus nerve. 1 , 2 , 97 Vagal sensory terminals in the cNTS appear to be topographically organized, such that input from the heart and lungs terminate in different subnuclei. 98 , 99 These vagal sensory neurons express a range of receptors and signalling molecules, recently mapped in detail by Bai et al. 100 using RNA sequencing data. Ultimately, this transcriptomic and anatomical specificity facilitates appropriate information flow from the viscera to the cNTS. 1 , 2 Additional sensory input to the NTS arises from the dorsal horn of the spinal cord, 12 , 101 perhaps relaying signals of tactile and nociceptive stimuli, although very little is known about these spinal inputs. These monosynaptic inputs from peripheral organs makes the NTS the primary brain region to receive and process rapid, neurochemical information regarding the internal environment of the body. Indeed, the cNTS is robustly activated in response to interoceptive stimuli. 4 , 6 , 92 Interestingly, however, the cNTS also receives widespread central inputs and is engaged following psychogenic stressors, suggesting that the cNTS is also sensitive to information regarding emotional states. 3 , 12 , 102
4.2. Central inputs to the cNTS
Central inputs to the NTS, which appear to be similar in rats and mouse, 11 are depicted in Figure 3 alongside efferent outputs. Below, I describe, in some detail, our current state of knowledge of the central inputs to the NTS. Of note, few studies have been able to limit the injection of retrograde tracers to the cNTS without significant leakage to more rostral areas or to the AP and DMV. This limitation makes it difficult to conclude with certainty which regions provide input to the cNTS specifically. In addition, many retrograde tracers, including the widely used CTb, are taken up by fibres of passage. In the case of the NTS, this could mean any descending projections to the spinal cord not terminating in the NTS may take up and transport CTb. 103 For a few brain regions, those limitations have been addressed by combining anterograde and retrograde tracing.
4.2.1. Telencephalic inputs
The insular, prelimbic and infralimbic cortices all provide significant bilateral input to the NTS in mice 11 , 12 and rats. 11 , 104 , 105 In rats, infralimbic neurons directly synapse onto catecholaminergic neurons in the NTS. 106 These descending inputs appear to mediate cortical modulation of sympathetic and parasympathetic activity 107 , 108 and, as such, may represent a functional link between emotional processing and autonomic outflow.
Subcortically, several regions of the extended amygdala innervate the NTS, including the central amygdala and bed nucleus of the stria terminalis of rat, 11 , 14 , 105 mouse 11 , 12 and rabbit. 109 Interestingly, all of these extended amygdala inputs appear to be exclusively ipsilateral. 11 Most input from the central amygdala arises from the medial subdivision in both rats 11 , 110 and mice, 12 although the lateral subnucleus also provides some synaptic input. 12 In the rat cNTS, central amygdala inputs terminate mostly in the medial and dorsomedial subnuclei and not only are predominantly GABAergic, 14 but also may release a range of neuropeptides as co‐transmitters, including nociceptin in mice 111 and somatostatin, neurotensin and vasoactive intestinal polypeptide in rats. 112
4.2.2. Diencephalic inputs
Arguably the densest central input to the NTS arises from the paraventricular nucleus of the hypothalamus (PVN), evidenced in rats 11 , 105 , 113 and mice. 11 , 12 This input is bilateral, 11 primarily originates in the more caudal parts of the PVN 114 (rat) and appears to represent a distinct parvocellular population, which does not overlap with neuroendocrine magnocellular PVN neurons in mice 11 and rats. 115 In rats, 60% of NTS‐projecting PVN neurons express the stress neuropeptide corticotropin‐releasing hormone (CRH), 114 and PVN CRH neurons project directly to the NTS in mice. 116 Evidence from rats suggests that a much smaller population (6%–10%) of NTS‐projecting PVN neurons express oxytocin, 114 , 117 PVN axons in the NTS express oxytocin, 118 and electrical stimulation of the PVN leads to release of oxytocin into the dorsal vagal complex. 119 In mice, PVN oxytocin cells do not provide significant direct synaptic input to the cNTS, although oxytocinergic fibres are clearly visible in the NTS of mice. 120 Finally, a subset of NTS‐projecting PVN neurons express the melanocortin 4 receptor. 121 Removal of this descending input from the PVN leads to the development of obesity, 122 although data from mice suggests this pathway has no effect on ad libitum feeding. 123 One possibility is that stress, a powerful stimulus to suppress eating in rodents, activates NTS‐projecting PVN neurons in mice, 12 which in turn mediate stress‐induced activation of cNTS neurons, including those that express catecholamines in rats. 124
Other hypothalamic inputs include the arcuate nucleus, the dorsomedial hypothalamus, and the lateral hypothalamus in both mice 11 , 12 and rats. 11 , 105 In addition, neurons in the ventromedial hypothalamus may innervate the NTS in mice, 125 although not every comprehensive study reported input from the dorsomedial and ventromedial hypothalamus in mouse. 11 , 12 Interestingly, descending input from the arcuate nucleus does not appear to arise from agouti‐related peptide or pro‐opiomelanocortin (POMC) neurons in mouse, 93 while in rat, evidence suggest a small population of POMC neurons do project to the dorsal vagal complex. 126
Finally, the parasubthalamic nucleus provides heavy, unilateral input to the NTS in mice 11 , 12 and rats, 11 a pathway that may mediate fear‐induced changes in autonomic outflow, 127 although studies of this particular nucleus are scarce. Indeed, the phenotype of these NTS‐projecting parasubthalamic neurons remains unknown, but may include tachykinin‐expressing, 128 CRH‐expressing 129 and/or glutamatergic neurons. 127
4.2.3. Mesencephalic and hindbrain inputs
The periaqueductal grey, Edinger–Westphal nucleus, parabrachial nucleus, Kölliker‐Fuse nucleus and Barrington's nucleus all provide direct input to the NTS in rats and mice. 11 , 12 , 130 , 131 Parabrachial input appears to mainly arise from glutamatergic, non‐calcitonin‐gene related peptide neurons in mice, 132 whereas tachykinin‐expressing neurons in the periaqueductal grey may be the source of input to the NTS in rats. 133 , 134 We recently found that NTS‐projecting Barrington's nucleus neurons are activated in response to acute restraint stress in mice and express the stress neuropeptide CRH, 12 supporting the idea that the NTS is engaged following psychogenic stimuli.
Finally, multiple lower brainstem regions provide input to the NTS in the mouse and rat, including the raphe obscurus, the raphe magnus, the reticular nucleus, the parapyramidal regions, the gigantocellular nucleus 11 , 12 and the DMV 135 (rats). Interestingly, input from the raphe magnus nucleus appears to partly mediate activation of cNTS neurons in response to interoceptive stressors, including LiCl, 136 and 5‐hydroxytryptamine signalling in the NTS (arising from either vagal afferents or the raphe nuclei) is an important modulator of central control of autonomic outflow 137 and feeding behaviour. 138
5. EFFERENT CONNECTIONS
Far from being a simple reflex station, which relays information from the afferent to the efferent vagus, the cNTS sends projections throughout the subcortical central nervous system, including to many autonomic control centres. 139 I was unable to find a comprehensive, analysis of the efferent connections of the mouse NTS based on injection of an anterograde tracer. However, some retrograde tracing in mouse has been reported for individual target regions and the findings from those studies will be included here when relevant. In addition, the anterograde mapping of specific subpopulations of cNTS neurons in transgenic mouse models does provide us with some idea of the outputs of the cNTS in mouse. One example of this type of anterograde tracing was carried out by Shi et al. 140 who mapped long‐range GABAergic inputs from the NTS.
5.1. Circumventricular organs
Neurons in the cNTS send projections to a number of sensory circumventricular organs: the AP, 141 the subfornical organ 142 and the vascular organ of laminar terminalis. 143 NTS input to the subfornical organ is inhibitory and may relay signals from peripheral baroreceptors in the rat. 144
5.2. Telencephalic projections
Notably, there is no evidence that the cortex or any of the hippocampal regions receive monosynaptic input from NTS neurons. Subcortically, the entire extended amygdala receives input from NTS neurons: The bed nucleus of the stria terminalis, the nucleus accumbens, the medial septum, the substantia innominata and the central amygdala are all synaptic targets of cNTS neurons in the rat. 31 , 143 , 145 , 146 At least a subset of NTS inputs to the bed nucleus of the stria terminalis in mice are GABAergic, 140 suggesting that this pathway is partly inhibitory, although other NTS cell types are known to project to these regions as well, including GLP‐1 neurons in mouse 35 and rat 143 and catecholaminergic neurons in rat, 146 but not NTS POMC neurons in mouse. 93
5.3. Diencephalic projections
Diencephalic targets include multiple regions in the hypothalamus. The PVN is a particularly densely innervated region in rats 139 , 143 , 147 and mice, 140 and the input is at least partly made up of GLP‐1 35 , 143 (mouse and rat), catecholaminergic 143 (rat), GABAergic 140 (mouse) and POMC fibres (mouse). 93 Other hypothalamic targets include the dorsomedial hypothalamus, the lateral hypothalamus and the arcuate nucleus. 143 , 147 These inputs are at least partly made up of GLP‐1 and catecholaminergic projections in the rat 143 and GABAergic 140 and GLP‐1 35 in the mouse. Additional diencephalic targets include the paraventricular thalamus and zona incerta in rat. 143 , 147
5.4. Mesencephalic and pontine projections
In the midbrain, the ventral tegmental area, the dorsal raphe and the periaqueductal grey all receive input from the NTS in the rat. 143 Further caudal, the Kölliker‐Fuse nucleus, parabrachial nucleus, locus coeruleus and Barrington's nucleus are targets of NTS efferents in rats. 143 Efferents to the parabrachial nucleus are assumed to drive suppression in appetite, 148 and, in the mouse, include input from POMC, 93 GLP‐1, 35 CCK 21 and noradrenergic neurons. 22 In the very caudal pons, the rostroventrolateral medulla 9 (cat) and DMV 9 , 149 (cat and rat) make up a subset of the brain‐wide autonomic control centres, which receive dense projections from the NTS. Finally, the neighbouring AP receives light input from the cNTS in the cat. 141
5.5. Spinal connections
In cats, the NTS projects to the thoracic ventral horn, the intermediolateral spinal column and phrenic motor neurons in the cervical spinal cord, suggesting some direct modulation of sympathetic outflow through spinal projections. 9 In mice, the trigeminal spinal nucleus and the principle sensory nucleus of the trigeminal receive dense input from GABAergic NTS neurons. 140
6. GLP‐1 NEURONS: A WIDELY‐PROJECTING SECOND‐ORDER POPULATION WITH DIVERSE MODULATORY ROLES
Although it is essential that we understand the anatomical connections of the cNTS as a whole, this nucleus is transcriptionally heterogenous and individual subpopulations of neurons are unlikely to serve identical functions or receive identical inputs (Table 1). In recent decades transgenic mouse models and viral gene transfer tools have facilitated investigations of the anatomy and function of anatomically and molecularly defined cell populations. Transgenic mice expressing Cre recombinase (Cre) under cell‐type specific promoters allow selective targeting using cre‐dependent viruses. As an example, ‐Cre transgenic mice express Cre under the control of the glucagon (Gcg) promoter. 150 Because GLP‐1 is also expressed under the Gcg promoter, this results in Cre expression selectively in GLP‐1 neurons in the lower brainstem and olfactory bulb (in addition to glucagon‐ and GLP‐1‐expressing cells in the periphery). 150 Injection of a cre‐dependent AAV into the dorsal vagal complex of these mice leads to expression of a desired transgene, often a chemogenetic receptor, an anterograde tracer or channelrhodopsin‐2 for functional and/or anatomical investigations.
Using these techniques, GLP‐1 neurons in the caudal brainstem are now relatively well understood, anatomically, cellularly and functionally. Here, I provide a very brief overview of their function and anatomy. Interested readers are referred to recent reviews on the subject for a more comprehension discussion. 92 , 102 , 151
6.1. GLP‐1 neurons: Distribution and innervation
Within the cNTS, GLP‐1 is expressed predominantly in glutamatergic neurons in the commissural, medial and ventral subnuclei in mice and rats, 35 , 36 and GLP‐1 neurons innervate widespread autonomic control centres, as well as nuclei involved in modulation of motivated behaviour in rats 143 and mice, 35 including the rostroventrolateral medulla, the PVN, dorsomedial hypothalamus and the bed nucleus of the stria terminalis. Whether specialized subpopulations of GLP‐1 neurons innervate distinct targets is still unknown, although classic tracing studies, demonstrating that 30%–40% of GLP‐1 neurons innervate distinct targets, would suggest some level of collateralization. 152 , 153 Use of retrogradely transported AAVs to target GLP‐1 neurons based on their projection target could reveal the extent of their collateralization. If anatomically distinct subpopulations exist, is it likely these are also functionally distinct? A previous finding indicating that stimulation of GLP‐1 receptors in the central amygdala increases anxiety‐like behaviour, whereas injection into the PVN decreases food intake without affecting anxiety‐like behaviour, would suggest at least some separation of functions. 154 However, it does not necessarily follow that specialized subpopulations exist. GLP‐1 neurons could simply modulate a range of diverse processes simultaneously. Future studies selectively manipulating subsets of GLP‐1 neurons based on their innervation targets should address these questions.
6.2. Monosynaptic inputs to GLP‐1 neurons
Until recently, mapping the monosynaptic inputs to molecularly defined cell populations was not possible. The recent development of Envelope‐A pseudotyped, G‐deleted Rabies virus (EnvA‐ΔG‐RABV) encoding GFP or mCherry represented a significant step forward. 155 In combination with Cre‐expressing transgenic mice or rats, this genetically modified rabies virus is efficient, exclusively retrograde, strictly monosynaptic and cell‐type specific. 155 Using such a EnvA‐ΔG‐RABV we recently mapped the monosynaptic inputs to GLP‐1 neurons in the mouse cNTS 12 and found that GLP‐1 neurons receive dense monosynaptic input from many of the same regions that provide input to the cNTS as a whole. Notable exceptions included cortical regions, the arcuate nucleus, the ventral tegmental area and the linear raphe nucleus. 12 We also identified polysynaptic inputs, including the hippocampal formation, the arcuate nucleus and the paraventricular thalamus. 12
6.3. Does anatomy predict function?
By mapping the inputs and outputs of subpopulations of NTS neurons we improve our understanding of their physiological functions with the interesting question often being: how do subpopulations differ anatomically? Unfortunately, we still have only few whole‐brain maps of molecularly defined, anatomically distinct subpopulations of NTS neurons: POMC and GLP‐1 neurons. 12 , 93 Based on these maps, we now know that the monosynaptic inputs to cNTS GLP‐1 and POMC neurons are similar with a few notable exceptions: There appears to be some, albeit limited, monosynaptic input from cortical regions to POMC neurons in the mouse, 93 and, although the lateral subnucleus of the central amygdala provides the majority of the input the GLP‐1 neurons, 12 POMC neurons receive their input from the medial subnucleus. 93 Considering that the lateral and medial subnuclei of the central amygdala have distinct inputs, outputs and expression profiles, 110 , 156 this could suggest POMC and GLP‐1 neurons form part of distinct brain circuits. Mapping these circuits is one step, although understanding their role in the modulation of behaviour and physiology is hampered by our difficulty in specifically manipulating subsets of neurons that provide direct synaptic inputs to molecularly defined cell types. The limiting factor has been the toxicity of rabies virus, leading to cell death within weeks of infection. 157 Further improvements in the toxicity of rabies viruses have been reported and may allow specific populations of input neurons to be manipulated for behavioural testing. 157
Regarding efferent connections, cNTS GLP‐1 neuron projections appear to be significantly more widespread than those of NTS POMC neurons in the mouse. 93 Although GLP‐1 neurons innervate multiple regions in the extended amygdala and hypothalamus, 35 , 143 the only forebrain regions to receive input from cNTS POMC neurons are the PVN, the PSTh, and the medial subnucleus of the central amygdala. 93 It will be interesting to determine whether these differences in inputs and outputs are matched by differences in function. Although both populations decrease food intake, an important functional difference appears to be their effect on heart rate: optogenetic activation of cNTS POMC neurons leads to a decrease in heart rate, 54 but chemogenetic activation of GLP‐1 neurons increases heart rate. 37 Interestingly, both GLP‐1 and POMC neurons are activated by solitary tract stimulation and CCK, 158 , 159 , 160 suggesting at least some overlap in the stimuli that engage them.
6.4. The importance of remembering the bigger picture in the study of single subpopulations of cNTS neurons
Peptidergic cNTS neurons, and perhaps GLP‐1 neurons in particular, 92 , 102 are exquisitely well‐positioned to integrate interoceptive or psychogenic signals. It is their anatomical configuration that enables this integration of multimodal signals of physical and mental well‐being. In turn, GLP‐1 neurons have the ability to impact truly varied processes both autonomic and behavioural (Table 1) and they are activated by both interoceptive (LiCl, gastric distension, large volume of food intake) and psychogenic stimuli (stress). 92 It is unknown whether individual GLP‐1 neurons drive one, some, or all of these functions (i.e., whether functional subpopulations of GLP‐1 neurons exist). Given that GLP‐1 neurons likely collateralize significantly (see above on distribution and innervation GLP‐1 neurons) we might speculate that individual GLP‐1 neurons drive multiple processes simultaneously. Importantly, it is likely that GLP‐1 neurons are not the only cNTS neurons with a very broad anatomical and functional profile (Table 1) and, when investigating the function of these other populations, we should keep in mind that they are not unlikely to modulate multiple downstream targets and, as a result, multiple physiological and behavioural processes simultaneously, as discussed for GLP‐1 neurons above. Examples of this ability to modulate multiple processes are provided in Table 1. Subpopulations of neurons do not work in isolation in the living organism and their artificial activation through chemo‐ or optogenetics is likely to have impact beyond the single output measured in most experiments. The cNTS is perhaps particularly sensitive to this as a result of its position as a link between sensory and emotional inputs, as well as its ability to modulate both behaviour and physiology.
7. CONCLUSIONS AND FUTURE DIRECTIONS
The afferent inputs to the NTS make it ideally suited to respond to both psychogenic and interoceptive stimuli, whereas its efferent connections facilitate widespread modulation of autonomic function and motivated behaviour. However, the specific circuits and cell types contributing to the functions of the NTS are still not fully understood. Future studies should take advantage of recently developed retrograde AAVs and the targeting of ChR2‐expressing terminals to selectively manipulate neurons, based on not only their neurochemical phenotype, but also their projection targets. These circuit‐ and cell type‐specific studies will, in combination with previously published classic knife‐cut and toxin studies, provide new insights into the functions of subpopulations of cNTS neurons.
CONFLICTS OF INTEREST
The author declares that he has no conflicts of interests.
8.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/jne.13132.
ACKNOWLEDGEMENTS
M. K. Holt is supported by a BHF Postdoctoral Fellowship (FS/IPBSRF/20/27001).
Holt MK. The ins and outs of the caudal nucleus of the solitary tract: An overview of cellular populations and anatomical connections. Journal of Neuroendocrinology. 2022;34(6):e13132. doi: 10.1111/jne.13132
Funding information British Heart Foundation, Grant/Award Number: FS/IPBSRF/20/27001
DATA AVAILABILITY
Data sharing is not applicable to this review because no new data were created or analyzed.
REFERENCES
- 1. Maniscalco JW, Rinaman L. Vagal interoceptive modulation of motivated behavior. Physiology (Bethesda). 2018;33(2):151‐167. doi: 10.1152/physiol.00036.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Berthoud HR, Albaugh VL, Neuhuber WL. Gut‐brain communication and obesity: understanding functions of the vagus nerve. J Clin Investig. 2021;131(10):e143770. doi: 10.1172/JCI143770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Maniscalco JW, Rinaman L. Interoceptive modulation of neuroendocrine, emotional, and hypophagic responses to stress. Physiol Behav. 2017;176:195‐206. doi: 10.1016/j.physbeh.2017.01.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Grill HJ, Hayes MR. Hindbrain neurons as an essential hub in the neuroanatomically distributed control of energy balance. Cell Metab. 2012;16(3):296‐309. doi: 10.1016/j.cmet.2012.06.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. MacDonald AJ, Ellacott KLJ. Astrocytes in the nucleus of the solitary tract: contributions to neural circuits controlling physiology. Physiol Behav. 2020;223:112982. doi: 10.1016/j.physbeh.2020.112982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Spyer KM, Gourine AV. Chemosensory pathways in the brainstem controlling cardiorespiratory activity. Philos Trans R Soc B Lond B Biol Sci. 2009;364(1529):2603‐2610. doi: 10.1098/rstb.2009.0082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schwaber JS, Kapp BS, Higgins GA, Rapp PR. Amygdaloid and basal forebrain direct connections with the nucleus of the solitary tract and the dorsal motor nucleus. J Neurosci. 1982;2(10):1424‐1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Whitehead MC. Neuronal architecture of the nucleus of the solitary tract in the hamster. J Comp Neurol. 1988;276(4):547‐572. doi: 10.1002/cne.902760409 [DOI] [PubMed] [Google Scholar]
- 9. Loewy AD, Burton H. Nuclei of the solitary tract: efferent projections to the lower brain stem and spinal cord of the cat. J Comp Neurol. 1978;181(2):421‐449. doi: 10.1002/cne.901810211 [DOI] [PubMed] [Google Scholar]
- 10. Ganchrow D, Ganchrow JR, Cicchini V, et al. Nucleus of the solitary tract in the C57BL/6J mouse: subnuclear parcellation, chorda tympani nerve projections, and brainstem connections. J Comp Neurol. 2014;522(7):1565‐1596. doi: 10.1002/cne.23484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gasparini S, Howland JM, Thatcher AJ, Geerling JC. Central afferents to the nucleus of the solitary tract in rats and mice. J Comp Neurol. 2020;528(16):2708‐2728. doi: 10.1002/cne.24927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Holt MK, Pomeranz LE, Beier KT, Reimann F, Gribble FM, Rinaman L. Synaptic inputs to the mouse dorsal vagal complex and its resident preproglucagon neurons. J Neurosci. 2019;39(49):9767‐9781. doi: 10.1523/JNEUROSCI.2145-19.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Herbert H, Moga MM, Saper CB. Connections of the parabrachial nucleus with the nucleus of the solitary tract and the medullary reticular formation in the rat. J Comp Neurol. 1990;293(4):540‐580. doi: 10.1002/cne.902930404 [DOI] [PubMed] [Google Scholar]
- 14. Saha S, Batten TF, Henderson Z. A GABAergic projection from the central nucleus of the amygdala to the nucleus of the solitary tract: a combined anterograde tracing and electron microscopic immunohistochemical study. Neuroscience. 2000;99(4):613‐626. [DOI] [PubMed] [Google Scholar]
- 15. Lynn RB, Hyde TM, Cooperman RR, Miselis RR. Distribution of bombesin‐like immunoreactivity in the nucleus of the solitary tract and dorsal motor nucleus of the rat and human: colocalization with tyrosine hydroxylase. J Comp Neurol. 1996;369(4):552‐570. doi: [DOI] [PubMed] [Google Scholar]
- 16. Li P, Janczewski WA, Yackle K, et al. The peptidergic control circuit for sighing. Nature. 2016;530(7590):293‐297. doi: 10.1038/nature16964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ellacott KLJ, Halatchev IG, Cone RD. Characterization of leptin‐responsive neurons in the caudal brainstem. Endocrinology. 2006;147(7):3190‐3195. doi: 10.1210/en.2005-0877 [DOI] [PubMed] [Google Scholar]
- 18. Fekete C, Wittmann G, Liposits Z, Lechan RM. Origin of cocaine‐ and amphetamine‐regulated transcript (CART)‐immunoreactive innervation of the hypothalamic paraventricular nucleus. J Comp Neurol. 2004;469(3):340‐350. doi: 10.1002/cne.10999 [DOI] [PubMed] [Google Scholar]
- 19. Garfield AS, Patterson C, Skora S, et al. Neurochemical characterization of body weight‐regulating leptin receptor neurons in the nucleus of the solitary tract. Endocrinology. 2012;153(10):4600‐4607. doi: 10.1210/en.2012-1282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zheng H, Patterson LM, Berthoud HR. CART in the dorsal vagal complex: sources of immunoreactivity and effects on Fos expression and food intake. Brain Res. 2002;957(2):298‐310. doi: 10.1016/S0006-8993(02)03640-5 [DOI] [PubMed] [Google Scholar]
- 21. Roman CW, Sloat SR, Palmiter RD. A tale of two circuits: CCKNTS neuron stimulation controls appetite and induces opposing motivational states by projections to distinct brain regions. Neuroscience. 2017;358:316‐324. doi: 10.1016/j.neuroscience.2017.06.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Roman CW, Derkach VA, Palmiter RD. Genetically and functionally defined NTS to PBN brain circuits mediating anorexia. Nat Commun. 2016;7:11905. doi: 10.1038/ncomms11905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cheng W, Gonzalez I, Pan W, et al. Calcitonin receptor neurons in the mouse nucleus tractus solitarius control energy balance via the non‐aversive suppression of feeding. Cell Metab. 2020;31(2):301‐312.e5. doi: 10.1016/j.cmet.2019.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. D'Agostino G, Lyons DJ, Cristiano C, et al. Appetite controlled by a cholecystokinin nucleus of the solitary tract to hypothalamus neurocircuit. Elife. 2016;5:e12225. doi: 10.7554/eLife.12225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Edwards CM, Strother J, Zheng H, Rinaman L. Amphetamine‐induced activation of neurons within the rat nucleus of the solitary tract. Physiol Behav. 2019;204:355‐363. doi: 10.1016/j.physbeh.2019.02.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Herbert H, Saper CB. Cholecystokinin‐, galanin‐, and corticotropin‐releasing factor‐like immunoreactive projections from the nucleus of the solitary tract to the parabrachial nucleus in the rat. J Comp Neurol. 1990;293(4):581‐598. doi: 10.1002/cne.902930405 [DOI] [PubMed] [Google Scholar]
- 27. Riche D, De Pommery J, Menetrey D. Neuropeptides and catecholamines in efferent projections of the nuclei of the solitary tract in the rat. J Comp Neurol. 1990;293(3):399‐424. doi: 10.1002/cne.902930306 [DOI] [PubMed] [Google Scholar]
- 28. Merchenthaler I. Corticotropin releasing factor (CRF)‐like immunoreactivity in the rat central nervous system. Extrahypothalamic distribution. Peptides. 1984;5:53‐69. doi: 10.1016/0196-9781(84)90265-1 [DOI] [PubMed] [Google Scholar]
- 29. Peng J, Long B, Yuan J, et al. A quantitative analysis of the distribution of CRH neurons in whole mouse brain. Front Neuroanat. 2017;11:11. doi: 10.3389/fnana.2017.00063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lee HS, Basbaum AI. Immunoreactive pro‐enkephalin and pro‐dynorphin products are differentially distributed within the nucleus of the solitary tract of the rat. J Comp Neurol. 1984;230(4):614‐619. doi: 10.1002/cne.902300409 [DOI] [PubMed] [Google Scholar]
- 31. Zardetto‐Smith AM, Gray TS. Organization of peptidergic and catecholaminergic efferents from the nucleus of the solitary tract to the rat amygdala. Brain Res Bull. 1990;25(6):875‐887. doi: 10.1016/0361-9230(90)90183-Z [DOI] [PubMed] [Google Scholar]
- 32. Do J, Chang Z, Sekerková G, McCrimmon DR, Martina M. A leptin‐mediated neural mechanism linking breathing to metabolism. Cell Rep. 2020;33(6):108358. doi: 10.1016/j.celrep.2020.108358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pérez SE, Wynick D, Steiner RA, Mufson EJ. Distribution of galaninergic immunoreactivity in the brain of the mouse. J Comp Neurol. 2001;434(2):158‐185. doi: 10.1002/cne.1171 [DOI] [PubMed] [Google Scholar]
- 34. Skofitsch G, Jacobowitz DM. Immunohistochemical mapping of galanin‐like neurons in the rat central nervous system. Peptides. 1985;6(3):509‐546. doi: 10.1016/0196-9781(85)90118-4 [DOI] [PubMed] [Google Scholar]
- 35. Llewellyn‐Smith IJ, Reimann F, Gribble FM, Trapp S. Preproglucagon neurons project widely to autonomic control areas in the mouse brain. Neuroscience. 2011;180:111‐121. doi: 10.1016/j.neuroscience.2011.02.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Larsen PJ, Tang‐Christensen M, Holst JJ, Orskov C. Distribution of glucagon‐like peptide‐1 and other preproglucagon‐derived peptides in the rat hypothalamus and brainstem. Neuroscience. 1997;77:257‐270. [DOI] [PubMed] [Google Scholar]
- 37. Holt MK, Cook DR, Brierley DI, et al. PPG neurons in the nucleus of the solitary tract modulate heart rate but do not mediate GLP‐1 receptor agonist‐induced tachycardia in mice. Mol Metab. 2020;39:101024. doi: 10.1016/j.molmet.2020.101024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gaykema RP, Newmyer BA, Ottolini M, et al. Activation of murine pre‐proglucagon‐producing neurons reduces food intake and body weight. J Clin Invest. 2017;127(3):1031‐1045. doi: 10.1172/JCI81335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Holt MK, Richards JE, Cook DR, et al. Preproglucagon neurons in the nucleus of the solitary tract are the Main source of brain GLP‐1, mediate stress‐induced Hypophagia, and limit unusually large intakes of food. Diabetes. 2019;68(1):21‐33. doi: 10.2337/db18-0729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Jin SL, Han VK, Simmons JG, Towle AC, Lauder JM, Lund PK. Distribution of glucagonlike peptide I (GLP‐I), glucagon, and glicentin in the rat brain: an immunocytochemical study. J Comp Neurol. 1988;271(4):519‐532. doi: 10.1002/cne.902710405 [DOI] [PubMed] [Google Scholar]
- 41. Liu J, Conde K, Zhang P, et al. Enhanced AMPA receptor trafficking mediates the anorexigenic effect of endogenous glucagon‐like peptide‐1 in the paraventricular hypothalamus. Neuron. 2017;96(4):897‐909.e5. doi: 10.1016/j.neuron.2017.09.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Merchenthaler I, Lane M, Shughrue P. Distribution of pre‐pro‐glucagon and glucagon‐like peptide‐1 receptor messenger RNAs in the rat central nervous system. J Comp Neurol. 1999;403:261‐280. [DOI] [PubMed] [Google Scholar]
- 43. Shi X, Chacko S, Li F, et al. Acute activation of GLP‐1‐expressing neurons promotes glucose homeostasis and insulin sensitivity. Mol Metab. 2017;6(11):1350‐1359. doi: 10.1016/j.molmet.2017.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tuesta LM, Chen Z, Duncan A, et al. GLP‐1 acts on habenular avoidance circuits to control nicotine intake. Nat Neurosci. 2017;20(5):708‐716. doi: 10.1038/nn.4540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Guggenberger M, Engster KM, Hofmann T, Rose M, Stengel A, Kobelt P. Cholecystokinin and bombesin activate neuronatin neurons in the nucleus of the solitary tract. Brain Res. 2020;1746:147006. doi: 10.1016/j.brainres.2020.147006 [DOI] [PubMed] [Google Scholar]
- 46. Mercer JG, Moar KM, Findlay PA, Hoggard N, Adam CL. Association of leptin receptor (OB‐Rb), NPY and GLP‐1 gene expression in the ovine and murine brainstem. Regul Pept. 1998;75‐76:271‐278. doi: 10.1016/S0167-0115(98)00078-0 [DOI] [PubMed] [Google Scholar]
- 47. Chen J, Cheng M, Wang L, et al. A vagal‐NTS neural pathway that stimulates feeding. Curr Biol. 2020;30(20):3986‐3998.e5. doi: 10.1016/j.cub.2020.07.084 [DOI] [PubMed] [Google Scholar]
- 48. Jennes L, Stumpf WE, Kalivas PW. Neurotensin: topographical distribution in rat brain by immunohistochemistry. J Comp Neurol. 1982;210(3):211‐224. doi: 10.1002/cne.902100302 [DOI] [PubMed] [Google Scholar]
- 49. Schroeder LE, Furdock R, Quiles CR, et al. Mapping the populations of neurotensin neurons in the male mouse brain. Neuropeptides. 2019;76:101930. doi: 10.1016/j.npep.2019.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bonnet MS, Pecchi E, Trouslard J, Jean A, Dallaporta M, Troadec JD. Central nesfatin‐1‐expressing neurons are sensitive to peripheral inflammatory stimulus. J Neuroinflammation. 2009;6(1):27. doi: 10.1186/1742-2094-6-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Bonnet MS, Ouelaa W, Tillement V, et al. Gastric distension activates NUCB2/nesfatin‐1‐expressing neurons in the nucleus of the solitary tract. Regul Pept. 2013;187:17‐23. doi: 10.1016/j.regpep.2013.10.001 [DOI] [PubMed] [Google Scholar]
- 52. Brailoiu GC, Dun SL, Brailoiu E, et al. Nesfatin‐1: distribution and interaction with a G protein‐coupled receptor in the rat brain. Endocrinology. 2007;148(10):5088‐5094. doi: 10.1210/en.2007-0701 [DOI] [PubMed] [Google Scholar]
- 53. Goebel‐Stengel M, Wang L, Stengel A, Taché Y. Localization of nesfatin‐1 neurons in the mouse brain and functional implication. Brain Res. 2011;1396:20‐34. doi: 10.1016/j.brainres.2011.04.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Cerritelli S, Hirschberg S, Hill R, Balthasar N, Pickering AE. Activation of brainstem pro‐opiomelanocortin neurons produces opioidergic analgesia, bradycardia and bradypnoea. PLoS One. 2016;11(4):e0153187. doi: 10.1371/journal.pone.0153187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Babic T, Townsend RL, Patterson LM, Sutton GM, Zheng H, Berthoud HR. Phenotype of neurons in the nucleus of the solitary tract that express CCK‐induced activation of the ERK signaling pathway. Am J Physiol Regul Integr Comp Physiol. 2009;296(4):R845‐R854. doi: 10.1152/ajpregu.90531.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Joseph SA, Pilcher WH, Bennett‐Clarke C. Immunocytochemical localization of ACTH perikarya in nucleus tractus solitarius: evidence for a second opiocortin neuronal system. Neurosci Lett. 1983;38(3):221‐225. doi: 10.1016/0304-3940(83)90372-5 [DOI] [PubMed] [Google Scholar]
- 57. Zhan C, Zhou J, Feng Q, et al. Acute and long‐term suppression of feeding behavior by POMC neurons in the brainstem and hypothalamus, respectively. J Neurosci. 2013;33(8):3624‐3632. doi: 10.1523/JNEUROSCI.2742-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Cheng W, Ndoka E, Maung JN, et al. NTS Prlh overcomes orexigenic stimuli and ameliorates dietary and genetic forms of obesity. Nat Commun. 2021;12(1):5175. doi: 10.1038/s41467-021-25525-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lee Y, Yang SP, Soares MJ, Voogt JL. Distribution of prolactin‐releasing peptide mRNA in the rat brain. Brain Res Bull. 2000;51(2):171‐176. doi: 10.1016/s0361-9230(99)00212-9 [DOI] [PubMed] [Google Scholar]
- 60. Morales T, Hinuma S, Sawchenko PE. Prolactin‐releasing peptide is expressed in afferents to the endocrine hypothalamus, but not in neurosecretory neurones. J Neuroendocrinol. 2000;12(2):131‐140. doi: 10.1046/j.1365-2826.2000.00428.x [DOI] [PubMed] [Google Scholar]
- 61. Pete G, Mack SO, Haxhiu MA, Walbaum S, Gauda EB. CO2‐induced c‐Fos expression in brainstem preprotachykinin mRNA containing neurons. Respir Physiol Neurobiol. 2002;130(3):265‐274. doi: 10.1016/S0034-5687(02)00013-0 [DOI] [PubMed] [Google Scholar]
- 62. Bailey TW, Appleyard SM, Jin YH, Andresen MC. Organization and properties of GABAergic neurons in solitary tract nucleus (NTS). J Neurophysiol. 2008;99(4):1712‐1722. doi: 10.1152/jn.00038.2008 [DOI] [PubMed] [Google Scholar]
- 63. Boychuk CR, Smith KC, Peterson LE, et al. A hindbrain inhibitory microcircuit mediates vagally‐coordinated glucose regulation. Sci Rep. 2019;9(1):2722. doi: 10.1038/s41598-019-39490-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Fong AY, Stornetta RL, Foley CM, Potts JT. Immunohistochemical localization of GAD67‐expressing neurons and processes in the rat brainstem: subregional distribution in the nucleus tractus solitarius. J Comp Neurol. 2005;493(2):274‐290. doi: 10.1002/cne.20758 [DOI] [PubMed] [Google Scholar]
- 65. Weston M, Wang H, Stornetta RL, Sevigny CP, Guyenet PG. Fos expression by glutamatergic neurons of the solitary tract nucleus after phenylephrine‐induced hypertension in rats. J Comp Neurol. 2003;460(4):525‐541. doi: 10.1002/cne.10663 [DOI] [PubMed] [Google Scholar]
- 66. Yamamoto K, Lalley P, Mifflin S. Acute intermittent optogenetic stimulation of nucleus tractus solitarius neurons induces sympathetic long‐term facilitation. Am J Physiol Regul Integr Comp Physiol. 2015;308(4):R266‐R275. doi: 10.1152/ajpregu.00381.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Aklan I, Sayar Atasoy N, Yavuz Y, et al. NTS catecholamine neurons mediate hypoglycemic hunger via medial hypothalamic feeding pathways. Cell Metab. 2020;31(2):313‐326.e5. doi: 10.1016/j.cmet.2019.11.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Machaalani R, Thawley M, Huang J, Chen H. Effects of prenatal cigarette smoke exposure on BDNF, PACAP, microglia and gliosis expression in the young male mouse brainstem. Neurotoxicology. 2019;74:40‐46. doi: 10.1016/j.neuro.2019.05.009 [DOI] [PubMed] [Google Scholar]
- 69. Shen L, Wang DQH, Xu M, Woods SC, Liu M. BDNF/Trk B signaling mediates the anorectic action of estradiol in the nucleus tractus solitarius. Oncotarget. 2017;8(48):84028‐84038. doi: 10.18632/oncotarget.21062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Resch JM, Fenselau H, Madara JC, et al. Aldosterone‐sensing neurons in the NTS exhibit state‐dependent pacemaker activity and drive sodium appetite via synergy with angiotensin II signaling. Neuron. 2017;96(1):190‐206.e7. doi: 10.1016/j.neuron.2017.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Fu C, Shi L, Wei Z, et al. Activation of Phox 2b‐expressing neurons in the nucleus tractus solitarii drives breathing in mice. J Neurosci. 2019;39(15):2837‐2846. doi: 10.1523/JNEUROSCI.2048-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Wang XF, Liu JJ, Xia J, Liu J, Mirabella V, Pang ZP. Endogenous glucagon‐like Peptide‐1 suppresses high‐fat food intake by reducing synaptic drive onto mesolimbic dopamine neurons. Cell Rep. 2015;12(5):726‐733. doi: 10.1016/j.celrep.2015.06.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Talman WT, Dragon DN, Ohta H, Lin LH. Nitroxidergic influences on cardiovascular control by NTS: a link with glutamate. Ann N Y Acad Sci. 2001;940(1):169‐178. doi: 10.1111/j.1749-6632.2001.tb03675.x [DOI] [PubMed] [Google Scholar]
- 74. Mohammed M, Johnson DN, Wang LA, et al. Targeting angiotensin type‐2 receptors located on pressor neurons in the nucleus of the solitary tract to relieve hypertension in mice. Cardiovasc Res. 2021;118:cvab 085‐cvab 896. doi: 10.1093/cvr/cvab085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Lamy CM, Sanno H, Labouebe G, et al. Hypoglycemia‐activated GLUT2 neurons of the nucleus tractus solitarius stimulate vagal activity and glucagon secretion. Cell Metab. 2014;19(3):527‐538. doi: 10.1016/j.cmet.2014.02.003 [DOI] [PubMed] [Google Scholar]
- 76. Cork SC, Richards JE, Holt MK, Gribble FM, Reimann F, Trapp S. Distribution and characterisation of glucagon‐like peptide‐1 receptor expressing cells in the mouse brain. Mol Metab. 2015;4(10):718‐731. doi: 10.1016/j.molmet.2015.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Card JP, Johnson AL, Llewellyn‐Smith IJ, et al. GLP‐1 neurons form a local synaptic circuit within the rodent nucleus of the solitary tract. J Comp Neurol. 2018;526(14):2149‐2164. doi: 10.1002/cne.24482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Cheng W, Ndoka E, Hutch CR, et al. Leptin receptor‐expressing nucleus tractus solitarius neurons suppress food intake independently of GLP1 in mice. JCI Insight. 2020;5(7):e134359. doi: 10.1172/jci.insight.134359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Yu H, Shi L, Chen J, et al. A neural circuit mechanism controlling breathing by leptin in the nucleus tractus solitarii. Neurosci Bull. 2022;38:149‐165. doi: 10.1007/s12264-021-00742-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. D'Agostino G, Lyons D, Cristiano C, et al. Nucleus of the solitary tract serotonin 5‐HT2C receptors modulate food intake. Cell Metab. 2018;28(4):619‐630.e5. doi: 10.1016/j.cmet.2018.07.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Stein LM, Lhamo R, Cao A, et al. Dorsal vagal complex and hypothalamic glia differentially respond to leptin and energy balance dysregulation. Transl Psychiatry. 2020;10(1):1‐12. doi: 10.1038/s41398-020-0767-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Reiner DJ, Mietlicki‐Baase EG, McGrath LE, et al. Astrocytes regulate GLP‐1 receptor‐mediated effects on energy balance. J Neurosci. 2016;36(12):3531‐3540. doi: 10.1523/JNEUROSCI.3579-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Hofmann GC, Hasser EM, Kline DD. Unilateral vagotomy alters astrocyte and microglial morphology in the nucleus tractus solitarii of the rat. Am J Physiol Regul Integr Comp Physiol. 2021;320(6):R945‐R959. doi: 10.1152/ajpregu.00019.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. MacDonald AJ, Holmes FE, Beall C, Pickering AE, Ellacott KLJ. Regulation of food intake by astrocytes in the brainstem dorsal vagal complex. Glia. 2020;68(6):1241‐1254. doi: 10.1002/glia.23774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Dallaporta M, Pecchi E, Pio J, Jean A, Horner KC, Troadec JD. Expression of leptin receptor by glial cells of the nucleus tractus solitarius: possible involvement in energy homeostasis. J Neuroendocrinol. 2009;21(1):57‐67. doi: 10.1111/j.1365-2826.2008.01799.x [DOI] [PubMed] [Google Scholar]
- 86. Chounlamountry K, Kessler JP. The ultrastructure of perisynaptic glia in the nucleus tractus solitarii of the adult rat: comparison between single synapses and multisynaptic arrangements. Glia. 2011;59(4):655‐663. doi: 10.1002/glia.21135 [DOI] [PubMed] [Google Scholar]
- 87. Accorsi‐Mendonca D, Zoccal DB, Bonagamba LG, Machado BH. Glial cells modulate the synaptic transmission of NTS neurons sending projections to ventral medulla of Wistar rats. Physiol Rep. 2013;1(4):e00080. doi: 10.1002/phy2.80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Vaughn AC, Cooper EM, DiLorenzo PM, et al. Energy‐dense diet triggers changes in gut microbiota, reorganization of gut‐brain vagal communication and increases body fat accumulation. Acta Neurobiol Exp (Wars). 2017;77(1):18‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Tadmouri A, Champagnat J, Morin‐Surun M. Activation of microglia and astrocytes in the nucleus tractus solitarius during ventilatory acclimatization to 10% hypoxia in unanesthetized mice. J Neurosci Res. 2014;92(5):627‐633. doi: 10.1002/jnr.23336 [DOI] [PubMed] [Google Scholar]
- 90. García‐Cáceres C, Balland E, Prevot V, et al. Role of astrocytes, microglia, and tanycytes in brain control of systemic metabolism. Nat Neurosci. 2019;22(1):7‐14. doi: 10.1038/s41593-018-0286-y [DOI] [PubMed] [Google Scholar]
- 91. Martinez D, Kline DD. The role of astrocytes in the nucleus tractus solitarii in maintaining central control of autonomic function. Am J Physiol Regul Integr Comp Physiol. 2021;320(4):R418‐R424. doi: 10.1152/ajpregu.00254.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Holt MK, Rinaman L. The role of nucleus of the solitary tract GLP1 and PrRP neurons in stress: anatomy, physiology, and cellular interactions. Br J Pharmacol. 2021;179:642‐658. doi: 10.1111/bph.15576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Wang D, He X, Zhao Z, et al. Whole‐brain mapping of the direct inputs and axonal projections of POMC and AgRP neurons. Front Neuroanat. 2015;9:40. doi: 10.3389/fnana.2015.00040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Dallaporta M, Himmi T, Perrin J, Orsini JC. Solitary tract nucleus sensitivity to moderate changes in glucose level. Neuroreport. 1999;10(12):2657‐2660. doi: 10.1097/00001756-199908200-00040 [DOI] [PubMed] [Google Scholar]
- 95. Paton JFR, Wang S, Polson JW, Kasparov S. Signalling across the blood brain barrier by angiotensin II: novel implications for neurogenic hypertension. J Mol Med. 2008;86(6):705‐710. doi: 10.1007/s00109-008-0324-4 [DOI] [PubMed] [Google Scholar]
- 96. Andresen MC, Yang MY. Non‐NMDA receptors mediate sensory afferent synaptic transmission in medial nucleus tractus solitarius. Am J Physiol. 1990;259(4 Pt 2):H1307‐H1311. doi: 10.1152/ajpheart.1990.259.4.H1307 [DOI] [PubMed] [Google Scholar]
- 97. Reis DJ, Granata AR, Perrone MH, Talman WT. Evidence that glutamic acid is the neurotransmitter of baroreceptor afferents terminating in the nucleus tractus solitarius (NTS). J Auton Nerv Syst. 1981;3(2):321‐334. doi: 10.1016/0165-1838(81)90073-4 [DOI] [PubMed] [Google Scholar]
- 98. Han W, Tellez LA, Perkins MH, et al. A neural circuit for gut‐induced reward. Cell. 2018;175(3):665‐678.e23. doi: 10.1016/j.cell.2018.08.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Barraco R, el‐Ridi M, Ergene E, Parizon M, Bradley D. An atlas of the rat subpostremal nucleus tractus solitarius. Brain Res Bull. 1992;29(6):703‐765. doi: 10.1016/0361-9230(92)90143-l [DOI] [PubMed] [Google Scholar]
- 100. Bai L, Mesgarzadeh S, Ramesh KS, et al. Genetic identification of vagal sensory neurons that control feeding. Cell. 2019;179(5):1129‐1143.e23. doi: 10.1016/j.cell.2019.10.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Menétrey D, Basbaum AI. Spinal and trigeminal projections to the nucleus of the solitary tract: a possible substrate for somatovisceral and viscerovisceral reflex activation. J Comp Neurol. 1987;255(3):439‐450. doi: 10.1002/cne.902550310 [DOI] [PubMed] [Google Scholar]
- 102. Holt MK. Mind affects matter: hindbrain GLP1 neurons link stress, physiology and behaviour. Exp Physiol. 2021;106:1853‐1862. doi: 10.1113/EP089445 [DOI] [PubMed] [Google Scholar]
- 103. Luppi PH, Fort P, Jouvet M. Iontophoretic application of unconjugated cholera toxin B subunit (CTb) combined with immunohistochemistry of neurochemical substances: a method for transmitter identification of retrogradely labeled neurons. Brain Res. 1990;534(1):209‐224. doi: 10.1016/0006-8993(90)90131-T [DOI] [PubMed] [Google Scholar]
- 104. Shipley MT. Insular cortex projection to the nucleus of the solitary tract and brainstem visceromotor regions in the mouse. Brain Res Bull. 1982;8(2):139‐148. doi: 10.1016/0361-9230(82)90040-5 [DOI] [PubMed] [Google Scholar]
- 105. van der Kooy D, Koda LY, McGinty JF, Gerfen CR, Bloom FE. The organization of projections from the cortex, amygdala, and hypothalamus to the nucleus of the solitary tract in rat. J Comp Neurol. 1984;224(1):1‐24. doi: 10.1002/cne.902240102 [DOI] [PubMed] [Google Scholar]
- 106. Gabbott PLA, Warner T, Busby SJ. Catecholaminergic neurons in medullary nuclei are among the post‐synaptic targets of descending projections from infralimbic area 25 of the rat medial prefrontal cortex. Neuroscience. 2007;144(2):623‐635. doi: 10.1016/j.neuroscience.2006.09.048 [DOI] [PubMed] [Google Scholar]
- 107. Owens NC, Sartor DM, Verberne AJM. Medial prefrontal cortex depressor response: role of the solitary tract nucleus in the rat. Neuroscience. 1999;89(4):1331‐1346. doi: 10.1016/S0306-4522(98)00389-3 [DOI] [PubMed] [Google Scholar]
- 108. Sévoz‐Couche C, Comet MA, Bernard JF, Hamon M, Laguzzi R. Cardiac baroreflex facilitation evoked by hypothalamus and prefrontal cortex stimulation: role of the nucleus tractus solitarius 5‐HT2A receptors. Am J Physiol Regul Integr Comp Physiol. 2006;291(4):R1007‐R1015. doi: 10.1152/ajpregu.00052.2006 [DOI] [PubMed] [Google Scholar]
- 109. Schwaber JS, Kapp BS, Higgins G. The origin and extent of direct amygdala projections to the region of the dorsal motor nucleus of the vagus and the nucleus of the solitary tract. Neurosci Lett. 1980;20(1):15‐20. [DOI] [PubMed] [Google Scholar]
- 110. Veening JG, Swanson LW, Sawchenko PE. The organization of projections from the central nucleus of the amygdala to brainstem sites involved in central autonomic regulation: a combined retrograde transport‐immunohistochemical study. Brain Res. 1984;303(2):337‐357. [DOI] [PubMed] [Google Scholar]
- 111. Hardaway JA, Halladay LR, Mazzone CM, et al. Central amygdala prepronociceptin‐expressing neurons mediate palatable food consumption and reward. Neuron. 2019;24:1037‐1052.e7. doi: 10.1016/j.neuron.2019.03.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Batten TFC, Gamboa‐Esteves FO, Saha S. Evidence for peptide co‐transmission in retrograde‐ and anterograde‐labelled central nucleus of amygdala neurones projecting to NTS. Auton Neurosci. 2002;98(1):28‐32. doi: 10.1016/S1566-0702(02)00026-7 [DOI] [PubMed] [Google Scholar]
- 113. Lynn RB, Kreider MS, Miselis RR. Thyrotropin‐releasing hormone‐immunoreactive projections to the dorsal motor nucleus and the nucleus of the solitary tract of the rat. J Comp Neurol. 1991;311(2):271‐288. doi: 10.1002/cne.903110208 [DOI] [PubMed] [Google Scholar]
- 114. Ruyle BC, Klutho PJ, Baines CP, Heesch CM, Hasser EM. Hypoxia activates a neuropeptidergic pathway from the paraventricular nucleus of the hypothalamus to the nucleus tractus solitarii. Am J Physiol Regul Integr Comp Physiol. 2018;315(6):R1167‐R1182. doi: 10.1152/ajpregu.00244.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Swanson LW, Sawchenko PE. Paraventricular nucleus: a site for the integration of neuroendocrine and autonomic mechanisms. Neuroendocrinology. 1980;31(6):410‐417. doi: 10.1159/000123111 [DOI] [PubMed] [Google Scholar]
- 116. Wang LA, Nguyen DH, Mifflin SW. Corticotropin‐releasing hormone projections from the paraventricular nucleus of the hypothalamus to the nucleus of the solitary tract increase blood pressure. J Neurophysiol. 2018;121(2):602‐608. doi: 10.1152/jn.00623.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Olson BR, Hoffman GE, Sved AF, Stricker EM, Verbalis JG. Cholecystokinin induces c‐fos expression in hypothalamic oxytocinergic neurons projecting to the dorsal vagal complex. Brain Res. 1992;569(2):238‐248. doi: 10.1016/0006-8993(92)90635-M [DOI] [PubMed] [Google Scholar]
- 118. Uchoa ET, Zahm DS, de Carvalho BB, Rorato R, Antunes‐Rodrigues J, Elias LLK. Oxytocin projections to the nucleus of the solitary tract contribute to the increased meal‐related satiety responses in primary adrenal insufficiency. Exp Physiol. 2013;98(10):1495‐1504. doi: 10.1113/expphysiol.2013.073726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Landgraf R, Malkinson T, Horn T, Veale WL, Lederis K, Pittman QJ. Release of vasopressin and oxytocin by paraventricular stimulation in rats. Am J Physiol Regul Integr Comp Physiol. 1990;258(1):R155‐R159. doi: 10.1152/ajpregu.1990.258.1.R155 [DOI] [PubMed] [Google Scholar]
- 120. Sutton AK, Pei H, Burnett KH, Myers MG, Rhodes CJ, Olson DP. Control of food intake and energy expenditure by Nos1 neurons of the paraventricular hypothalamus. J Neurosci. 2014;34(46):15306‐15318. doi: 10.1523/JNEUROSCI.0226-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Blevins JE, Morton GJ, Williams DL, et al. Forebrain melanocortin signaling enhances the hindbrain satiety response to CCK‐8. Am J Physiol Regul Integr Comp Physiol. 2009;296(3):R476‐R484. doi: 10.1152/ajpregu.90544.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Kirchgessner AL, Sclafani A. PVN‐hindbrain pathway involved in the hypothalamic hyperphagia‐obesity syndrome. Physiol Behav. 1988;42(6):517‐528. doi: 10.1016/0031-9384(88)90153-9 [DOI] [PubMed] [Google Scholar]
- 123. Stachniak TJ, Ghosh A, Sternson SM. Chemogenetic synaptic silencing of neural circuits localizes a hypothalamus→midbrain pathway for feeding behavior. Neuron. 2014;82(4):797‐808. doi: 10.1016/j.neuron.2014.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Dayas CV, Buller KM, Day TA. Hypothalamic paraventricular nucleus neurons regulate medullary catecholamine cell responses to restraint stress. J Comp Neurol. 2004;478(1):22‐34. doi: 10.1002/cne.20259 [DOI] [PubMed] [Google Scholar]
- 125. Lindberg D, Chen P, Li C. Conditional viral tracing reveals that steroidogenic factor 1‐positive neurons of the dorsomedial subdivision of the ventromedial hypothalamus project to autonomic centers of the hypothalamus and hindbrain. J Comp Neurol. 2013;521(14):3167‐3190. doi: 10.1002/cne.23338 [DOI] [PubMed] [Google Scholar]
- 126. Zheng H, Patterson LM, Phifer CB, Berthoud HR. Brain stem melanocortinergic modulation of meal size and identification of hypothalamic POMC projections. Am J Physiol Regul Integr Comp Physiol. 2005;289(1):R247‐R258. doi: 10.1152/ajpregu.00869.2004 [DOI] [PubMed] [Google Scholar]
- 127. Liu C, Lee CY, Asher G, et al. Posterior subthalamic nucleus (PSTh) mediates innate fear‐associated hypothermia in mice. Nat Commun. 2021;12(1):2648. doi: 10.1038/s41467-021-22914-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Goto M, Swanson LW. Axonal projections from the parasubthalamic nucleus. J Comp Neurol. 2004;469(4):581‐607. doi: 10.1002/cne.11036 [DOI] [PubMed] [Google Scholar]
- 129. Zhu X, Krasnow SM, Roth‐Carter QR, et al. Hypothalamic signaling in anorexia induced by indispensable amino acid deficiency. Am J Physiol Endocrinol Metab. 2012;303(12):E1446‐E1458. doi: 10.1152/ajpendo.00427.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Krukoff TL, Harris KH, Jhamandas JH. Efferent projections from the parabrachial nucleus demonstrated with the anterograde tracer Phaseolus vulgaris leucoagglutinin. Brain Res Bull. 1993;30(1):163‐172. doi: 10.1016/0361-9230(93)90054-F [DOI] [PubMed] [Google Scholar]
- 131. Saper CB, Loewy AD. Efferent connections of the parabrachial nucleus in the rat. Brain Res. 1980;197(2):291‐317. doi: 10.1016/0006-8993(80)91117-8 [DOI] [PubMed] [Google Scholar]
- 132. Huang D, Grady FS, Peltekian L, Laing JJ, Geerling JC. Efferent projections of CGRP/Calca‐expressing parabrachial neurons in mice. J Comp Neurol. 2021;529(11):2911‐2957. doi: 10.1002/cne.25136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Boscan P, Paton JFR. Excitatory convergence of periaqueductal gray and somatic afferents in the solitary tract nucleus: role for neurokinin 1 receptors. Am J Physiol Regul Integr Comp Physiol. 2005;288(1):R262‐R269. doi: 10.1152/ajpregu.00328.2004 [DOI] [PubMed] [Google Scholar]
- 134. Lucas LR, Hurley DL, Krause JE, Harlan RE. Localization of the tachykinin neurokinin B precursor peptide in rat brain by immunocytochemistry and in situ hybridization. Neuroscience. 1992;51(2):317‐345. doi: 10.1016/0306-4522(92)90318-V [DOI] [PubMed] [Google Scholar]
- 135. Rinaman L, Card JP, Schwaber JS, Miselis RR. Ultrastructural demonstration of a gastric monosynaptic vagal circuit in the nucleus of the solitary tract in rat. J Neurosci. 1989;9(6):1985‐1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Leon RM, Borner T, Stein LM, et al. Activation of PPG neurons following acute stressors differentially involves hindbrain serotonin in male rats. Neuropharmacology. 2021;187:108477. doi: 10.1016/j.neuropharm.2021.108477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Ramage AG, Villalón CM. 5‐Hydroxytryptamine and cardiovascular regulation. Trends Pharmacol Sci. 2008;29(9):472‐481. doi: 10.1016/j.tips.2008.06.009 [DOI] [PubMed] [Google Scholar]
- 138. Wu Q, Clark MS, Palmiter RD. Deciphering a neuronal circuit that mediates appetite. Nature. 2012;483(7391):594‐597. doi: 10.1038/nature10899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Ricardo JA, Tongju KE. Anatomical evidence of direct projections from the nucleus of the solitary tract to the hypothalamus, amygdala, and other forebrain structures in the rat. Brain Res. 1978;153(1):1‐26. doi: 10.1016/0006-8993(78)91125-3 [DOI] [PubMed] [Google Scholar]
- 140. Shi MY, Ding LF, Guo YH, Cheng YX, Bi GQ, Lau PM. Long‐range GABAergic projections from the nucleus of the solitary tract. Mol Brain. 2021;14(1):38. doi: 10.1186/s13041-021-00751-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Shapiro RE, Miselis RR. The central neural connections of the area postrema of the rat. J Comp Neurol. 1985;234(3):344‐364. doi: 10.1002/cne.902340306 [DOI] [PubMed] [Google Scholar]
- 142. Zardetto‐Smith AM, Gray TS. A direct neural projection from the nucleus of the solitary tract to the subfornical organ in the rat. Neurosci Lett. 1987;80(2):163‐166. doi: 10.1016/0304-3940(87)90647-1 [DOI] [PubMed] [Google Scholar]
- 143. Rinaman L. Ascending projections from the caudal visceral nucleus of the solitary tract to brain regions involved in food intake and energy expenditure. Brain Res. 2010;1350:18‐34. doi: 10.1016/j.brainres.2010.03.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Shioya M, Tanaka J. Inputs from the nucleus of the solitary tract to subfornical organ neurons projecting to the paraventricular nucleus in the rat. Brain Res. 1989;483(1):192‐195. doi: 10.1016/0006-8993(89)90054-1 [DOI] [PubMed] [Google Scholar]
- 145. Gerfen CR, Sawchenko PE. An anterograde neuroanatomical tracing method that shows the detailed morphology of neurons, their axons and terminals: Immunohistochemical localization of an axonally transported plant lectin, Phaseolus vulgaris leucoagglutinin (PHA‐L). Brain Res. 1984;290(2):219‐238. doi: 10.1016/0006-8993(84)90940-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Bienkowski MS, Rinaman L. Common and distinct neural inputs to the medial central nucleus of the amygdala and anterior ventrolateral bed nucleus of stria terminalis in rats. Brain Struct Funct. 2013;218(1):187‐208. doi: 10.1007/s00429-012-0393-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Horst GJT, De Boer P, Luiten PGM, Van Willigen JD. Ascending projections from the solitary tract nucleus to the hypothalamus. A Phaseolus vulgaris lectin tracing study in the rat. Neuroscience. 1989;31(3):785‐797. doi: 10.1016/0306-4522(89)90441-7 [DOI] [PubMed] [Google Scholar]
- 148. Palmiter RD. The parabrachial nucleus: CGRP neurons function as a general alarm. Trends Neurosci. 2018;41(5):280‐293. doi: 10.1016/j.tins.2018.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Norgren R. Projections from the nucleus of the solitary tract in the rat. Neuroscience. 1978;3(2):207‐218. doi: 10.1016/0306-4522(78)90102-1 [DOI] [PubMed] [Google Scholar]
- 150. Parker HE, Adriaenssens A, Rogers G, et al. Predominant role of active versus facilitative glucose transport for glucagon‐like peptide‐1 secretion. Diabetologia. 2012;55:2445‐2455. doi: 10.1007/s00125-012-2585-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Trapp S, Brierley DI. Brain GLP‐1 and the regulation of food intake: GLP‐1 action in the brain and its implications for GLP‐1 receptor agonists in obesity treatment. Br J Pharmacol. 2022;179:557‐570. doi: 10.1111/bph.15638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Alhadeff AL, Rupprecht LE, Hayes MR. GLP‐1 neurons in the nucleus of the solitary tract project directly to the ventral tegmental area and nucleus accumbens to control for food intake. Endocrinology. 2012;153:647‐658. doi: 10.1210/en.2011-1443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Llewellyn‐Smith IJ, Marina N, Manton RN, Reimann F, Gribble FM, Trapp S. Spinally projecting preproglucagon axons preferentially innervate sympathetic preganglionic neurons. Neuroscience. 2015;284:872‐887. doi: 10.1016/j.neuroscience.2014.10.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Kinzig KP, D'Alessio DA, Herman JP, et al. CNS glucagon‐like peptide‐1 receptors mediate endocrine and anxiety responses to interoceptive and psychogenic stressors. J Neurosci. 2003;23(15):6163‐6170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Wall NR, Wickersham IR, Cetin A, De La Parra M, Callaway EM. Monosynaptic circuit tracing in vivo through Cre‐dependent targeting and complementation of modified rabies virus. Proc Natl Acad Sci U S A. 2010;107(50):21848‐21853. doi: 10.1073/pnas.1011756107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. McCullough KM, Morrison FG, Hartmann J, Carlezon WA, Ressler KJ. Quantified coexpression analysis of central amygdala subpopulations. eNeuro. 2018;5:ENEURO.0010‐18.2018. doi: 10.1523/ENEURO.0010-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Chatterjee S, Sullivan HA, Mac Lennan BJ, et al. Nontoxic, double‐deletion‐mutant rabies viral vectors for retrograde targeting of projection neurons. Nat Neurosci. 2018;21(4):638‐646. doi: 10.1038/s41593-018-0091-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Hisadome K, Reimann F, Gribble FM, Trapp S. CCK stimulation of GLP‐1 neurons involves α1‐adrenoceptor‐mediated increase in glutamatergic synaptic inputs. Diabetes. 2011;60:2701‐2709. doi: 10.2337/db11-0489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Hisadome K, Reimann F, Gribble FM, Trapp S. Leptin directly depolarizes preproglucagon neurons in the nucleus tractus solitarius: electrical properties of glucagon‐like peptide 1 neurons. Diabetes. 2010;59:1890‐1898. doi: 10.2337/db10-0128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Appleyard SM, Bailey TW, Doyle MW, et al. Proopiomelanocortin neurons in nucleus tractus solitarius are activated by visceral afferents: regulation by cholecystokinin and opioids. J Neurosci. 2005;25(14):3578‐3585. doi: 10.1523/JNEUROSCI.4177-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this review because no new data were created or analyzed.


