FIGURE 2.
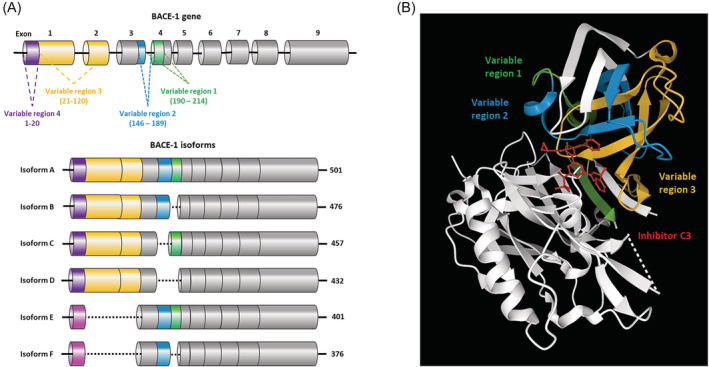
Alternative splicing of BACE1 produces different isoforms. (A) BACE1 is alternatively spliced at four variable regions; 190–214 (green), 146–189 (blue), 21–120 (yellow), and 1–20 (purple). Alternative splicing of these regions produces at least six distinct isoforms, the most characterized being Isoforms A–F: Isoform A (501aa), Isoform B (476aa), Isoform C (457aa), Isoform D (432aa), Isoform E (401aa), Isoform F (376aa) are depicted. Isoforms E and F contain an alternative Exon 1 (pink). (B) The variable regions shown on the BACE1 crystal structure with inhibitor C3 (red) made using structure 3TPR, on RSCB Protein DataBank (https://www.rcsb.org/). Variable regions 1 (190–214) (green), 2 (146–189) (blue), and 3 (21–120) (yellow) show close proximity to the inhibitor binding site. The structure is missing 62 residues, including variable region 1–20, which therefore could not be labeled
