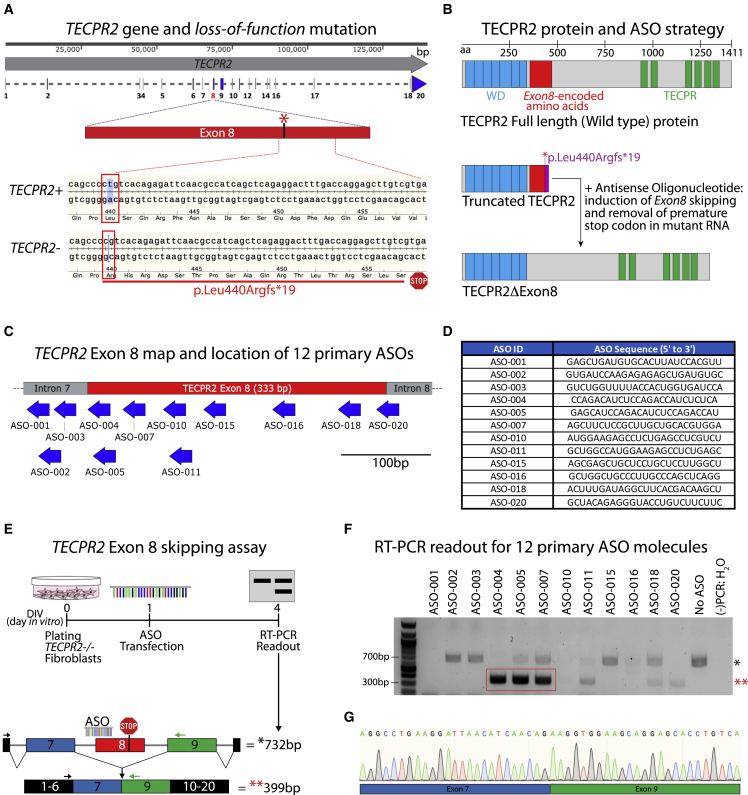
Figure 1.
TECPR2 gene and identification of ASO sequences inducing TECPR2 exon 8 skipping
(A) Map of TECPR2 gene showing exon/intron regions and highlighting exon 8 wild-type TECPR2+ and mutant TECPR2- (c.1319delT, p.Leu440Argfs∗19) alleles. The SPG49 patient is homozygous for the mutation (TECPR2-/-). (B) Diagram of TECPR2 protein, highlighting annotated WD domain (predicted for residues 23–343) and TECPR repeat domain (predicted for residues 945–1353), along with the protein region (in red) encoded by TECPR2 exon 8, which corresponds to 333 bp (111 amino acids). The premature stop codon in the SPG49 patient presumably results in truncated protein. ASO-mediated induction of TECPR2 exon 8 skipping could restore expression of a shorter form of TECPR2 protein lacking exon 8-encoded sequence (TECPR2ΔExon8). (C) Diagram of TECPR2 exon 8 and surrounding intron 7 and 8 regions showing location of initial set of 12 ASOs designed to induce exon 8 skipping. (D) Table listing ASO ID and sequence of initial set of 25-mer (25 nucleotides in length) ASOs used in primary screening. For these ASOs, all bases had 2′-O-methyl modification and all linkages were phosphorothioate chemistry. (E) Experimental outline for TECPR2 exon 8 skipping assay using TECPR2-/- patient-derived fibroblasts. Enrichment of a shorter (minus 333 bp) PCR amplicon of ∼399 bp is expected if TECPR2 exon 8 skipping is induced. (F) DNA gel inverse image with results of initial screening of 12 primary ASOs showing that treatment of TECPR2-/- patient fibroblasts with ASO-004, ASO-005, and ASO-007 can lead to significant enrichment of ∼399-bp PCR amplicon (highlighted in red rectangle and with red asterisks [∗∗]), corresponding to TECPR2 transcript minus exon 8. (−)PCR:H2O, water was used as negative control for PCR assay. (G) Sanger sequencing chromatogram of purified lower PCR amplicon (∼399 bp) obtained from samples treated with ASO-005, demonstrating TECPR2 exon 8 skipping with precise splicing of exon 7 and exon 9 sequences.