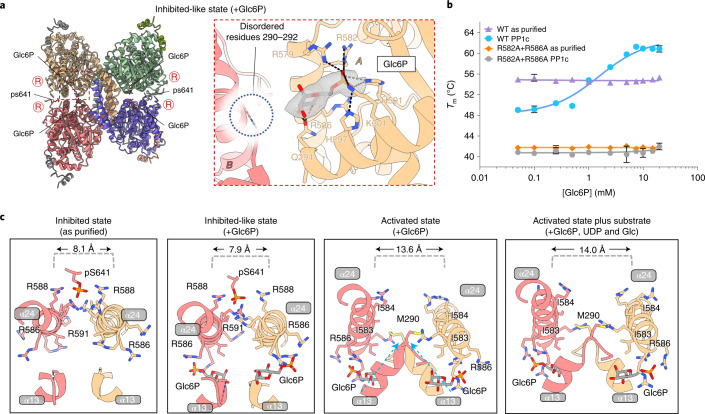
Fig. 5. Phosphorylation hinders transition into the activated (R) state as shown by the phosphorylated inhibited (T) state bound to Glc6P.
a, Overall model of the phosphorylated T state bound to Glc6P and the interactions with this activator. Inset shows cryo-EM density for Glc6P. Arginine clusters-containing regulatory α24 helices are labelled ‘R’. b, Thermal shift assay of as-purified (phosphorylated) versus PP1c-treated (dephosphorylated) GYS1–GYG1ΔCD (labelled WT) and GYS1p.R582A+p.R586A–GYG1ΔCD (labelled R582A + R586A) complexes in the presence of increasing concentrations of Glc6P. Median melting temperatures and standard deviations are shown (n = 4 technical repeats). c, Regulatory helix interactions and conformational changes as seen in our cryo-EM structures. Key residues are labelled. Distances between the regulatory α24 helices were determined as the distances between the Cα atoms of the Asn587 residues.