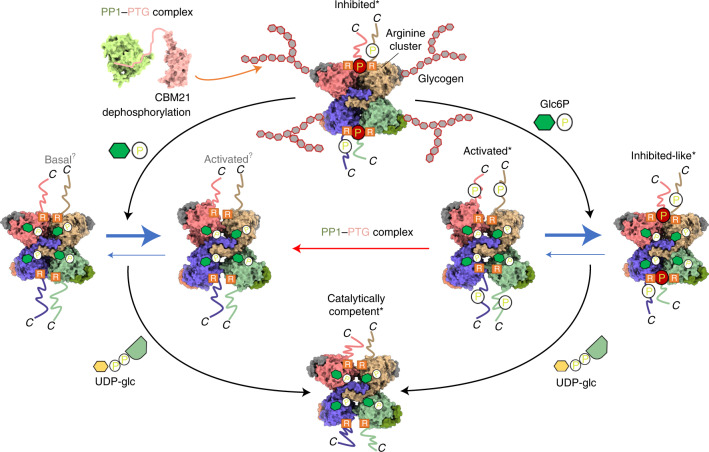
Fig. 7. Proposed model of phosphorylation and Glc6P regulation of GYS1 activity.
Only the C termini and 3a phosphorylation site are shown for simplicity. In addition, the associated glycogen is only shown for the inhibited state, although it is present in all other states. Asterisk indicates structures that have been experimentally determined. Question mark indicates theoretical structures. Our model based on the structural data proposes that the inhibited (T) state is catalytically inactive because the phosphorylated N and C termini bind to a subunit interface. This locking interaction reduces GYS1 flexibility and prevents active site closure by the two Rossmann domains. Glc6P binding to the allosteric site overcomes these inhibitory effects to promote a conformational change to the activated (R) state. However, the R state is in a dynamic equilibrium with an inhibited-like state, owing to competition between the locking interactions of phosphorylated termini at the subunit interface and the conformational change due to Glc6P binding. The inhibition of phosphorylation can also be relieved by the concerted actions of the PP1–PTG complex that binds to the associated glycogen and dephosphorylates the GYS1 N and C termini, resulting in the basal (I) state. This intermediate state is more susceptible to the allosteric effects of Glc6P binding, shifting the dynamic equilibrium more toward the activated state. In the activated state, binding of the substrate UDP-glc promotes the closure of the cleft between the two Rossmann domains, resulting in a catalytically competent state for extending the associated glycogen chain.