Abstract
Background
Hepatocellular carcinoma (HCC) is rapidly increasing in the U.S. and is a leading cause of mortality for patients with cirrhosis. Discovering novel biomarkers for risk stratification of HCC is paramount. We examined biomarkers of the gut-liver axis in a prospective multicenter cohort.
Methods
Patients with cirrhosis without a history of HCC were recruited between May 2015 and March 2020 and prospectively followed at 3 tertiary care hospitals in Los Angeles. Microbiome analysis was performed on duodenal biopsies and metabolomic analysis was performed on serum samples, collected at the time of enrollment. Optimal microbiome-based survival analysis and Cox proportional hazards regression analysis were used to determine microbiota and metabolite associations with HCC development, respectively.
Results
A total of 227 participants with liver cirrhosis contributed a total of 459.58 person-years of follow-up, with 14 incident HCC diagnoses. Male sex (HR = 7.06, 95% CI = 1.02–54.86) and baseline hepatic encephalopathy (HE, HR = 4.65, 95% CI = 1.60–13.52) were associated with developing HCC over follow-up. Adjusting for age, sex, baseline HE, and alkaline phosphatase, an increased risk of HCC were observed for participants with the highest versus lowest three quartiles for duodenal Alloprevotella (HR = 3.22, 95% CI = 1.06–9.73) and serum taurocholic acid (HR = 6.87, 95% CI = 2.32–20.27), methionine (HR = 9.97, 95% CI = 3.02–32.94), and methioninesulfoxide (HR = 5.60, 95% CI = 1.84–17.10). Being in the highest quartile for Alloprevotella or methionine had a sensitivity and specificity for developing HCC of 85.71% and 60.56%, respectively, with an odds ratio of 10.92 (95% CI = 2.23–53.48).
Conclusion
Alloprevotella and methionine, methioninesulfoxide, and taurocholic acid predicted future HCC development in a high-risk population of participants with liver cirrhosis.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10620-021-07299-2.
Keywords: Bile acids, Biogenic amines, Alloprevotella, Methionine, Taurocholic acid, Time-to-event, Small intestine
Introduction
Hepatocellular carcinoma (HCC) is a highly lethal cancer with a significant increasing incidence and mortality [1]. For a person with cirrhosis, the 5-year risk of developing could be as high as 30% [2, 3]. Therefore, given the high burden of disease, early detection of HCC is critical and is associated with improved survival [4]. Unfortunately, there is currently a lack of accurate and easily accessible biomarkers for the early detection of HCC.
There has been growing evidence that the intestinal microbiome contributes to the progression of cirrhosis and cancer pathogenesis [5–7]. While most human studies have focused on fecal analyses, fecal analysis alone may miss important taxa and functional changes limited to the small intestine (SI). The microbial population, structure, and function vary considerably throughout the gut [8], and prior research suggests that the microbiome of the SI, the site of key physiological functions including bile acid uptake and nutrient absorption [9, 10], is distinct from the fecal microbiome of the colon [8]. The liver is the end point of all portal circulation with the majority of its tributaries arising from the upper and middle gastrointestinal tract. While the SI microbiome has been associated with significant clinical outcomes in other diseases [11], it has not been assessed in HCC.
In addition to the microbiome, there is also a growing body of literature that has shown microbe-associated metabolites to be important biomarkers and affecters of liver disease and cancer [12, 13]. Microbe-associated metabolites such as bile acids, damage-associated molecular patterns (DAMPs), amino acids, and short-chain fatty acids can be released into the portal circulation and absorbed in the liver causing changes in immune function, cytokine release, and cell signaling [13–15]. Therefore, the study of these microbe-associated metabolites is just as important as the study of the microbes themselves.
This study examines for the first time the association between the duodenal microbiome and related metabolites in association with HCC risk in a multicenter prospective cohort of participants with cirrhosis. We report a novel microbial and metabolite signature that is associated with HCC risk.
Methods
Study Population and Data Collection
The Microbiome, Microbial Markers & Liver Disease (M3LD) study is an ongoing multicenter prospective cohort study of participants with cirrhosis designed to investigate the etiology and biomarkers for HCC. Eligibility criteria for M3LD include patients who are 18 years or older with a diagnosis of cirrhosis confirmed by biopsy or imaging. Those who have received a solid organ transplant or a diagnosis of HCC are excluded. Participants were consented and enrolled at the time of their standard of care screening or surveillance esophagogastroduodenoscopy (EGD) between May 2015 and March 2020 at three clinical sites: Cedars-Sinai Medical Center (CS), Ronald Reagan UCLA Medical Center (UC), and the VA Greater Los Angeles Healthcare System (VA). Demographic information and clinical data were abstracted from the participant’s record using case report forms that were standardized across all clinical sites. HCC diagnosis was confirmed by biopsy or by cross-sectional imaging using Liver Reporting & Data System (LI-RADS) criteria for HCC. The study design was approved by each site’s respective Institutional Review Board. Written informed consent was obtained for all participants.
Specimen Collection
Four (approximately 1–2 cm in size) duodenal biopsies were collected during esophagogastroduodenoscopy (EGD) being performed as part of routine medical care for esophageal variceal screening/surveillance at the baseline study visit. The biopsies were obtained from the 2nd portion of the duodenum, approximately 2–5 cm distal to the ampulla of Vater. Peripheral blood specimens were also collected at the baseline visit. The biopsies were obtained before any aspiration of oral or gastric contents to minimize cross-contamination with other sections of the gastrointestinal tract. The biopsies were immediately flash-frozen in individual cryovials using ethanol and dry ice and stored at − 80 °C until DNA extraction. Serum, plasma, and PBMC were archived and stored at − 80 °C until testing. Following collection, specimens from all sites were transported to a central repository for secure storage until testing. All vacutainers, cryovials, and specimen bags were prelabeled with barcodes linked to the study participant to ensure tracking accuracy.
Microbiome Sequencing
DNA was extracted using the ZymoBIOMICS DNA Microprep Kit (Zymo Research, Irvine, CA, USA) per the manufacturer’s protocol. The V4 region of the 16S ribosomal RNA gene was amplified by PCR and underwent 250 × 2 paired-end sequencing on an Illumina NovaSeq (Illumina, San Diego, CA, USA).
The sequences were processed using the DADA2 pipeline in R which assigns taxonomy using the SILVA 132 database. After pre-processing in R, the data were incorporated into QIIME 2 version 2019.10. Amplicon sequence variants were filtered if not present in at least 15% of all samples. Sequence depths ranged from 6193 to 422,641 per sample.
Serum Metabolites
An untargeted profile of metabolites (n = 186) and bile acids (n = 16) were measured in archived sera by the NIH West Coast Metabolomics Center (Davis, CA). For the biogenic amines, the hydrophilic interaction liquid chromatography (HILIC) analysis workflow involved analysis of polar phase of lipid extraction by ultra-high pressure liquid chromatography (UHPLC) on a Waters BEH Amide Column, interfaced to a SCIEX Triple TOF 6600 mass spectrometer (high resolution, accurate mass), with a 16.8 min total run time. Extraction was carried out using a bi-phasic solvent system of cold methanol, methyl tert-butyl ether (MTBE), and water. Dried extracts were resuspended in acetonitrile. The LC/QTOFMS analyses were performed using an Agilent 1290 Infinity LC system (G4220A binary pump, G4226A autosampler, and G1316C Column Thermostat) coupled to a SCIEX Triple TOF mass spectrometer. Data were collected in both positive and negative ion modes and analyzed using MS DIAL, open software for metabolome analysis.
For the bile acids, serum samples were spiked with internal standards of bile acids. Reverse-phase liquid chromatography was performed on a Waters Acquity BEH C18 column (1.7 µm, 2.1 × 100 mm) with its corresponding Vanguard precolumn at 45 °C at a flow rate of 400 µL/min. Extracts were analyzed by liquid chromatography (Waters ACQUITY UPLC I-Class system) coupled to a Sciex 6500 + QTRAP hybrid, triple quadrupole linear ion trap mass spectrometer. Scheduled multiple reaction monitoring (MRM) was performed with optimized collision energies, de-clustering potentials, and collision cell exit potentials for the individual analytes. All analytes were quantified against 6-point calibration curves using internal standards. Software Analyst 1.6.3 and MultiQuant 3.0.2 (AB Sciex) were used for data acquisition and quantification, including peak integration, peak area computation, and for computing the molar concentrations for the analytes by using calibration curves created using internal standards. Results are reported as a concentration unit (nanomolar) of the analyte extracted.
Statistical Analyses
Descriptive Analyses
Baseline demographic and clinical data were analyzed to see if there were differences between patients who developed HCC as compared to those who did not. Differences between groups were examined using analysis of variance (ANOVA) for continuous variables and Fisher’s exact test for categorical variables. Baseline demographic and clinical variables that were associated with HCC with a p < 0.1 were included as adjustment factors for the microbiome and metabolites.
Duodenal microbiome diversity and composition were examined in association with HCC development. Beta diversity was calculated using the DEICODE plugin in QIIME 2, which employs a robust Aitchison analysis that accounts for the sparse compositional nature of microbiome data [16]. Beta diversity was modeled for association with HCC development by adjusting for covariates using the adonis package in R, which implements a permutational analysis of variances utilizing distance matrices. Beta diversity was visualized using principal components analysis. Alpha diversity was measured using Shannon Index, a measurement of species evenness and richness, with data rarefied to 6192 sequences [17]. Alpha diversity was tested using analysis of variance in R. Differential abundance testing was performed using DESEq2 in R, which employs a Bayesian approach to fit non‐rarified count data to a negative binomial model [18]. P-values were converted to q-values to correct for multiple hypothesis testing and a q-value ≤ 0.05 was deemed significant [19].
Serum metabolite and bile acid levels were natural log transformed and examined for association with development of HCC using analysis of variance (ANOVA), corrected for multiple hypothesis testings using the false discovery rate (FDR) [20]. Metabolites achieving an adjusted p-value < 0.05 were deemed statistically significant and visualized using notch box plots.
Time-to-Event Analyses
Time-to-event analyses were conducted to examine the association between baseline characteristics and biomarkers and time to HCC diagnosis. Follow-up time was defined by the date of the baseline study visit to exit date defined as the date of diagnosis of HCC, death, liver transplantation, loss to follow-up, or censoring at the end of the follow-up period, whichever came first. For demographic and clinical variables, Cox proportional hazard regression models were used to estimate hazard ratios (HR) and 95% confidence intervals (CI). Demographic and clinical variables that had a p < 0.1 in the univariate models were entered into a multivariate analysis along with age (base model).
For microbiota, time-to-event analyses were performed using the optimal microbiome-based survival analysis using linear and non-linear bases of operational taxonomic units (OMiSALN) performed using the R package OMiSA [21] OMiSALN is a semi-parametric test based on variance-component score and re-sampling methods. Given the large number of microbiota observed in our population, only genera or families that had an unadjusted p-value of 0.05 by DESEq2 (described in the Descriptive Analysis section) were assessed for time-to-event association with HCC using OMiSALN, and all microbiota with a p < 0.05 in OMiSALN were deemed statistically significant. For each microbe achieving a p < 0.05 in OMiSALN, HRs and 95% CIs were calculated using Cox proportional hazard regression models for quartiles of biomarker levels, with the highest quartile being compared to the lower three quartiles, adjusted for the clinical and demographic variables from the base model.
For serum metabolites, HRs and 95% CIs were calculated using this Cox base model for each biomarker (in quartiles) and corrected for multiple hypothesis testing using FDR. Metabolites achieving an adjusted p-value < 0.05 were deemed statistically significant.
Selected metabolites and microbiota that were deemed to be significantly associated with HCC in the time-to-event models were visualized using Kaplan–Meier curves with levels in the highest quartile versus the lowest three quartiles tested using the log-rank test and included in a multi-biomarker Cox proportional hazard regression model. Similarly, those same selected metabolites and microbiota quartiles were used to determine the odds of developing HCC via logistic regression analysis adjusting for covariates. All authors had access to the study data and reviewed and approved the final manuscript.
Results
Descriptive Analyses
A total 227 participants were included in this study with 459.58 person-years of follow-up time. Over the follow-up period, 14 participants were diagnosed with an incident HCC, 21 patients died, and 24 patients received a liver transplant. Of the 14 participants that were diagnosed with HCC, 6 ultimately received a liver transplant with all 6 explants confirming a diagnosis of HCC on pathology. Twelve of the 14 incident HCC cases met Milan criteria (one lesion less ≤ 5 cm, or up to 3 lesions each with diameter ≤ 3 cm, and without extrahepatic involvement or major vessel involvement). The average follow-up time per participant was 2.02 years. More males developed HCC than females (92.9% versus 66.2%, p = 0.04), Table 1. The prevalence of hepatic encephalopathy (HE) was greater among participants who went on to develop HCC (50% versus 24.9%, p = 0.04). AFP levels were low at baseline and the median MELD score was 9.8.
Table 1.
Select baseline participant characteristics by HCC development
| Total n = 227 | Did not Develop HCC (n = 213) | Developed HCC (n = 14) | p-value |
|---|---|---|---|
| Mean (SD) or n (%) | Mean (SD) or n (%) | ||
| Age (yr) (mean) | 59.4 (12.4) | 61.4 (10.0) | 0.60 |
| Site | |||
| CS | 96 (45.1) | 9 (64.3) | 0.37 |
| UC | 55 (25.8) | 2 (14.3) | |
| VA | 62 (29.1) | 3 (21.4) | |
| Sex | |||
| Male | 141 (66.2) | 13 (92.9) | 0.04 |
| Female | 72 (33.8) | 1 (7.1) | |
| Race | |||
| White | 177 (83.1) | 11 (78.6) | 0.60 |
| African American | 18 (8.5) | 2 (14.3) | |
| Asian | 11 (5.2) | 1 (7.1) | |
| Other | 7 (3.3) | 0 (0.0) | |
| Ethnicity | |||
| Non-Hispanic/Latinx | 134 (62.9) | 9 (64.3) | 1.00 |
| Hispanic or Latinx | 79 (37.1) | 5 (35.7) | |
| Etiology of Cirrhosis | |||
| HCV | 66 (31.0) | 7 (50.0) | 0.49 |
| Alcohol | 56 (26.3) | 2 (14.3) | |
| HBV | 6 (2.8) | 1 (7.1) | |
| NAFLD | 46 (21.6) | 2 (14.3) | |
| Other | 39 (18.3) | 2 (14.3) | |
| Complication of cirrhosis | |||
| HE | |||
| No | 160 (75.1) | 7 (50.0) | 0.04 |
| Yes | 53 (24.9) | 7 (50.0) | |
| Varices | |||
| No | 86 (40.4) | 4 (28.6) | 0.26 |
| Yes | 127 (59.6) | 10 (71.4) | |
| Ascites | |||
| No | 119 (55.9) | 8 (57.1) | 1.00 |
| Yes | 94 (44.1) | 6 (42.9) | |
| At Least 1 Complication | |||
| No | 61 (28.6) | 3 (21.4) | 0.76 |
| Yes | 152 (71.4) | 11 (78.6) | |
| Diabetes | |||
| No | 161 (75.6) | 11 (78.6) | 1.00 |
| Yes | 52 (24.4) | 3 (21.4) | |
| AFP (ng/mL) (mean) | 5.13 (21.96) | 4.25 (3.56) | 0.88 |
| MELD (mean) | 9.7 (6.1) | 10.9 (7.8) | 0.46 |
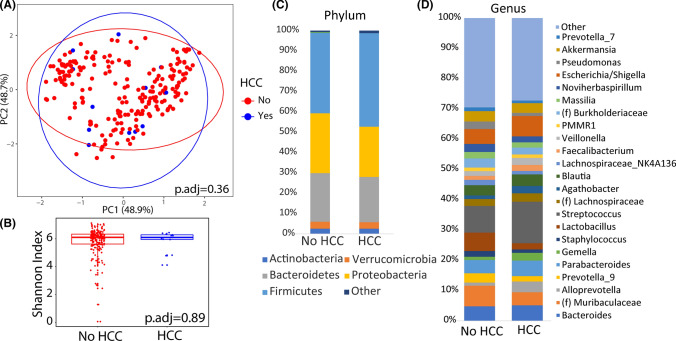
After adjustment for demographic and clinical variables that were significantly associated with HCC status (sex and baseline HE), there were no baseline differences in the gut microbiome between participants who developed HCC by beta diversity (p.adj = 0.36) or alpha diversity (p.adj = 0.89), Fig. 1a, b. Differential abundance testing on a family and genus level did not show any taxa that were different in patients who developed HCC after additional adjustment for multiple hypothesis testing, Fig. 1c, d, Supplemental Tables 1, and 2. However, the family Bacillacea, Christensenellaceae, and Lactobacillaceae were all underrepresented in participants who developed HCC compared to participants who did not with unadjusted p-values < 0.05. Additionally, six genera were underrepresented (Lachnospiraceae UCG-010, CAG-352, Peptoclostridium, Catenibacterium, Prevotellaceae NK3B31 group, and Actinobacillus) or overrepresented (Listeria, Alloprevotella, Anaerostipes, and Gemella) in participants who developed HCC with unadjusted p-values < 0.05.
Fig. 1.
Baseline duodenal microbial differences between patients who developed hepatocellular carcinoma (HCC) as compared to those that never did. a Principal coordinate analysis plot of microbial composition colored by HCC development. b Alpha diversity by Shannon Index of the microbial community between patients that developed HCC as compared to those that did not. Taxonomic plots by c phyla and genus d between patients who did develop HCC as compared to those that did not. Only taxa that had a relative abundance of ≥ 1% are shown
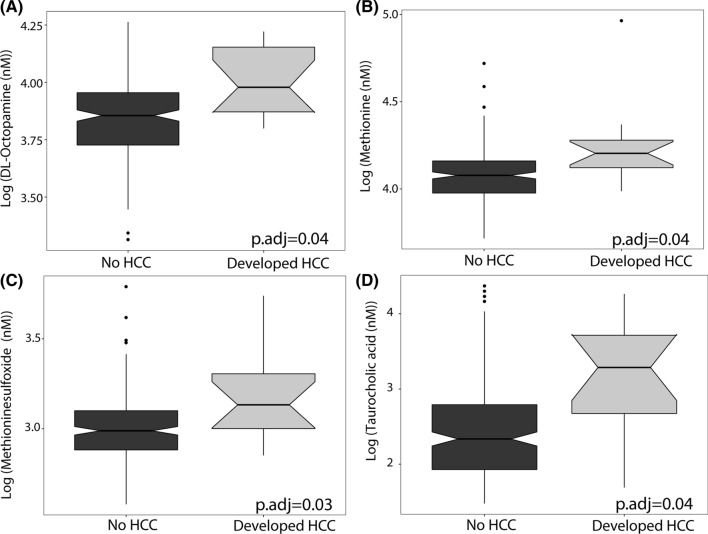
The overall metabolite composition between those that developed HCC versus those that never did were not significantly different as seen by the principal component analysis plot in supplemental Fig. 1 (p.adj = 0.10). However, of the 186 biogenic amines assayed, three biogenic amines were associated with HCC status after adjustment for multiple hypothesis testing: DL-Octopamine (p.adj = 0.04), Methionine (p.adj = 0.04), and Methioninesulfoxide (p.adj = 0.03). All three of these metabolites were higher among participants who developed HCC (Fig. 2). Of the 16 bile acids tested, taurocholic acid (TCA) was observed in higher concentrations in participants who developed HCC (p.adj = 0.04, Fig. 2). A complete list of biogenic amines and bile acids with their FDR-adjusted p-value for their association with HCC is shown in Supplemental Tables 3 and 4.
Fig. 2.
Notch box plots of metabolites that were statistically different between patients that developed hepatocellular carcinoma (HCC) as compared to those that never developed HCC. P-values listed are adjusted for sex, age, baseline HE, alkaline phosphatase, and false discovery rate
Time-to-Event Analyses
Univariate Cox-proportional hazards regression analysis showed that female sex, baseline HE and baseline alkaline phosphatase (AlkPhos) were associated with HCC risk, with a p-value < 0.1 (Table 2). In multivariate regression models including age, sex, HE, and AlkPhos, female sex (HR = 0.14; 95% CI = 0.02–0.99) and HE (HR = 4.65; 95% CI = 1.60–13.52) were significantly associated with HCC risk. All further time-to-event analyses were adjusted for age, sex, baseline HE, and AlkPhos.
Table 2.
Hazard ratios and 95% confidence intervals for the association between baseline characteristics and risk of HCC development over the follow-up period
| Variable | Univariate cox-proportional hazards regression | Multivariate cox-proportional hazards regression | ||||
|---|---|---|---|---|---|---|
| Hazard Ratio | 95% Confidence Interval | p-value | Hazard Ratio | 95% Confidence Interval | p-value | |
| Site | ||||||
| CS | 1.00 | (reference category) | – | – | – | |
| UC | 0.41 | 0.09–1.90 | 0.25 | – | – | – |
| VA | 0.61 | 0.16–2.26 | 0.46 | – | – | – |
| Age | 1.01 | 0.97–1.06 | 0.57 | 1.02 | 0.97–1.07 | 0.55 |
| Sex | ||||||
| Male | 1.00 | (reference category) | – | – | – | |
| Female | 0.15 | 0.02–1.12 | 0.06 | 0.14 | 0.02–0.99 | 0.05 |
| Race | ||||||
| White | 1.00 | (reference category) | – | – | – | |
| African American | 1.54 | 0.34–6.94 | 0.58 | – | – | – |
| Asian | 1.43 | 0.18–11.11 | 0.73 | – | – | – |
| Other | NE | – | – | – | ||
| Ethnicity | – | – | ||||
| Non-Hispanic/Latinx | 1.00 | (reference category) | – | – | – | |
| Hispanic or Latinx | 1.06 | 0.36–3.17 | 0.91 | – | – | – |
| Etiology of cirrhosis | ||||||
| HCV | 1.00 | (reference category) | - | – | – | |
| Alcohol | 0.39 | 0.08–1.89 | 0.24 | – | – | – |
| HBV | 1.48 | 0.18–12.06 | 0.71 | – | – | – |
| NAFLD | 0.45 | 0.09–2.16 | 0.32 | – | – | – |
| Other | 0.52 | 0.11–2.49 | 0.41 | – | – | – |
| Complication of cirrhosis | ||||||
| Hepatic encephalopathy | ||||||
| No | 1.00 | (reference category) | – | – | – | |
| Yes | 3.12 | 1.093–8.895 | 0.03 | 4.65 | 1.60–13.52 | 0.01 |
| Varices | ||||||
| No | 1.00 | (reference category) | – | – | – | |
| Yes | 1.76 | 0.56–5.66 | 0.33 | |||
| Ascites | ||||||
| No | 1.00 | (reference category) | – | – | – | |
| Yes | 0.993 | 0.344–2.86 | 0.99 | – | – | – |
| Diabetes | ||||||
| No | 1.00 | (reference category) | – | – | – | |
| Yes | 0.861 | 0.240–3.088 | 0.82 | – | – | – |
| AFP (ng/mL) | 0.996 | 0.965–1.03 | 0.84 | – | – | – |
| MELD | 1.034 | 0.955–1.121 | 0.41 | – | – | – |
| AlkPhos (IU/l) | 0.987 | 0.974–1.001 | 0.07 | 0.99 | 0.97–1.00 | 0.11 |
| AST (IU/l) | 1.004 | 0.994–1.014 | 0.47 | – | – | – |
| ALT (IU/l) | 0.994 | 0.972–1.018 | 0.65 | – | – | – |
| Bilirubin (mg/dL) | 1.059 | 0.925–1.212 | 0.41 | – | – | – |
| INR | 1.89 | 0.413–8.62 | 0.41 | – | – | – |
| Creatinine (mg/dL) | 0.426 | 0.098–1.836 | 0.25 | – | – | – |
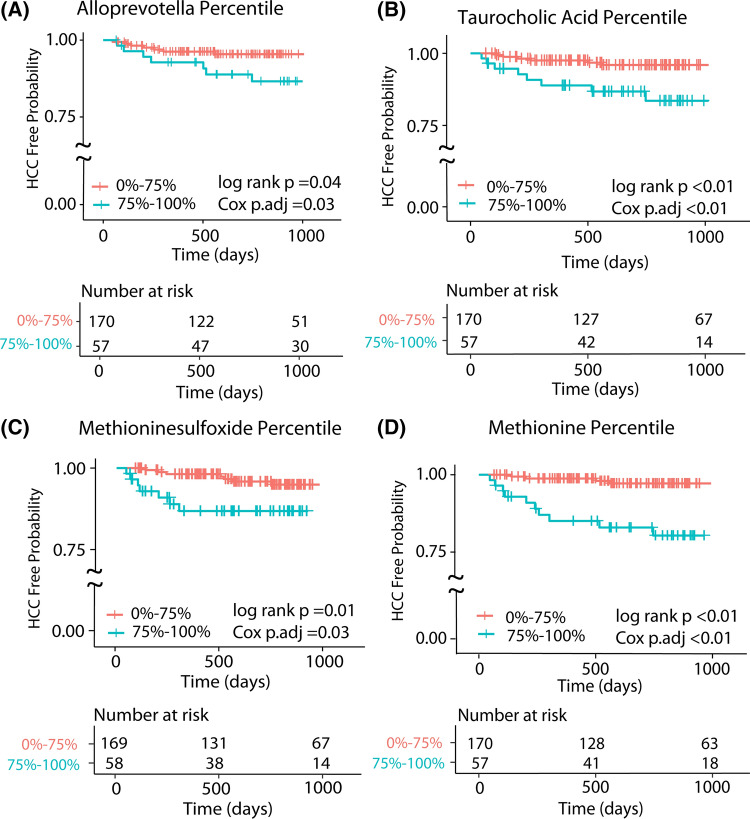
In the microbiome time-to-event analyses, Alloprevotella was the only genus that was significantly associated with HCC development by OMiSALN. When incorporated into the Cox regression analysis, participants with the highest quartile of the genus Alloprevotella, had an increased risk of HCC compared to participants with levels in the lower three quartiles (HR = 3.22, 95% CI = 1.06–9.73; log-rank p = 0.04), Table 3 and Fig. 3. Of all the biogenic amines examined, participants with the highest versus lowest three quartiles for methionine and methioninesulfoxide concentrations were at increased risk of HCC (HR = 9.97, 95% CI = 3.02–32.94 and HR = 5.60, 95% CI = 1.84–17.10, respectively). TCA was the only bile acid associated with HCC; participants with the highest quartile of TCA concentration were at increased risk of HCC (HR = 6.87; 95% CI = 2.32–20.27), Table 3 and Fig. 3. A complete list of metabolites and bile acids and their univariate associations with time to HCC are shown in Supplemental Tables 5 and 6.
Table 3.
Hazard ratios and 95% confidence intervals for the association between baseline biomarker levels and risk of HCC development over the follow-up period
| Variable | Single Biomarker Cox- Proportional Hazards Regressiona | Multiple Biomarker Cox-Proportional Hazards Regressionb | ||||
|---|---|---|---|---|---|---|
| Hazard Ratio | 95% Confidence Interval | p-value | Hazard Ratio | 95% Confidence Interval | p-value | |
| Microbes | ||||||
| Alloprevotella Q4 | 3.22 | 1.06–9.73 | 0.04 | 2.31 | 0.76–7.02 | 0.14 |
| Biogenic amines | ||||||
| Methionine Q4 | 9.97 | 3.02–32.94 | < 0.01 | 8.76 | 2.62–29.33 | <0.01 |
| Methioninesulfoxide Q4 | 5.60 | 1.84–17.10 | < 0.01 | – | – | – |
| Bile acids | ||||||
| Taurocholic acid Q4 | 6.87 | 2.32–20.27 | < 0.01 | – | – | – |
aModels adjusted for age, sex, HE, and AlkPhos; b Models adjusted for age, sex, HE, AlkPhos, and all biomarkers in the table. Q4: quartile 4 (i.e. 75–100%) versus quartiles 1–3 (reference)
Fig. 3.
Kaplan Meir Curves of variables associated with increased risk of HCC development by Cox Regression adjusting for false discovery rate and age, sex, and the presence of baseline hepatic encephalopathy. a Kaplan Meir curve of duodenal Alloprevotella comparing the upper quartile to the bottom 3 quartile. b Kaplan Meir curve of duodenal taurocholic acid (serum bile acid) comparing the upper quartile to the bottom 3 quartile. c Kaplan Meir curve of duodenal methioninesulfoxide (serum metabolite) comparing the upper quartile to the bottom 3 quartile. d Kaplan Meir curve of duodenal methionine (serum metabolite) comparing the upper quartile to the bottom 3 quartile
Ad hoc proportional relationship analysis showed that methionine, methioninesulfoxide, and TCA levels were highly correlated with one another (p < 0.001). In the multimarker Cox proportional hazards regression model, we included only one of these metabolites that displayed the strongest association with HCC in the univariate model (methionine), along with Alloprevotella, age, sex, baseline HE, and AlkPhos. After mutual adjustment, the Alloprevatella relationship with HCC risk was slightly attenuated (HR = 2.31, 95% CI = 0.76–7.02), as was the association between methionine and HCC risk (HR = 8.76, 95% CI = 2.62–29.33).
Being in the highest quartile for either Alloprevotella or methionine had a sensitivity of 85.71% and a specificity of 60.56% for developing HCC (Supplemental Table 7). Adjusting for age, sex, baseline HE, and AlkPhos, being in the highest quartile for either Alloprevotella or methionine had an odds ratio of 10.92 for developing HCC (95% CI 2.23–53.48) by logistic regression.
Discussion
This is the largest study to date examining the duodenal microbiome and circulating biogenic amines and bile acids in a prospective cohort of patients with cirrhosis. In our study of 227 participants, we show that duodenal levels of Alloprevotella and serum concentrations of serum methionine, methioninesulfoxide, and TCA were significantly associated with HCC risk.
In our base clinical and demographic time-to-event model, we found that male sex and HE were significantly associated with increased risk of HCC. Research has shown that males have a higher HCC risk compared to females for a multitude of reasons [22]. Men are more likely to be exposed to environmental risk factors for HCC such as alcohol and smoking [23]. Men are also more likely to have metabolic derangements, such as diabetes and metabolic syndrome, which are among the most dominant risk factors for HCC [24]. There is also recent evidence that estrogen may play a protective role in the development of HCC [22]. Therefore, our finding that males have a significantly higher risk of developing HCC is consistent with the previous published works.
HE, which is typically a late complication of cirrhosis, has a strong link to dysbiosis and small intestinal bacterial overgrowth [29, 30]. The observed association between HE and HCC risk may be related to common associations with alterations in the microbiome. Additionally, HE may be a marker for participants with more advanced cirrhosis who have increased cumulative risk for HCC [23].
Our observation that Alloprevotella was associated with HCC risk adds to a growing body of literature demonstrating an important role for the gut microbiome in HCC development [5]. Liu et al. showed an increase in fecal Alloprevotella in patients with hepatitis B-related HCC as compared to healthy controls [25]. Our study suggests that the SI microbiome may also be an important compartment for HCC risk. The exact role of Alloprevotella in the development of HCC is still unknown, but some studies have suggested an association of Alloprevotella with gut barrier function [26]. This suggests that Alloprevotella in the SI may affect gut permeability and increase bacterial translocation, metabolite transport, or bile acid exposure to the liver.
There is a growing body of evidence stating the importance of metabolomics profiling in both liver disease and cancer [12, 13]. Given the growing recognition for the importance of metabolic activity of gut microbiota in HCC pathogenesis, we examined biogenic amines and bile acid concentration in relation to HCC risk. We observed that participants with higher levels of serum methionine, methioninesulfoxide, and TCA were at increased risk for developing HCC. Methionine is an essential amino acid that plays a role in several key pathways such as protein synthesis and DNA methylation [27]. In normal everyday processes, methionine is either used for protein synthesis or converted to S-adenosylmethionine (SAM) where it is further used for DNA methylation, protein methylation, or as a component of homocysteine, cysteine, taurine, and adenosine monophosphate [27]. Several cancer types have been shown to be dependent on methionine or have deficiency in methionine conversion. Cancer cells that are deficient in these pathways are unable to regenerate methionine in either the de novo or salvage pathways, making them dependent on methionine [27]. Several animal studies have shown a diet that is restricted in methionine is protective against cancer growth [28]. In regards to HCC, studies have shown that HCC harbors defects that lead to an inability to convert methionine to SAM [29]. SAM has several anti-tumor properties which include antioxidative effects, stabilization of DNA repair, and changes in DNA methylation [29]. Additionally, studies have shown that addition of SAM can lead to reduced tumorigenesis and metastasis [30, 31]. Therefore, the increase in serum methionine in patients who developed HCC may be secondary to their inability to covert methionine to SAM effectively. Consistent with these findings, epidemiological human studies have shown that patients on vegetable-based diets, which are low in methionine, have a reduce risk of HCC development [32]
Serum TCA was also seen as a key risk factor for the development of HCC. TCA is a primary bile acid and studies have consistently shown that high levels of bile acids can induce liver injury, hepatocyte DNA damage, and hepatocyte apoptosis [33]. Elevated levels of TCA in the systemic circulatory system likely represent elevated levels of TCA in the liver and portal circulation. TCA in the colon can be converted to deoxycholic acid and hydrogen sulfide by bacterial communities, a tumor promoter and carcinogen, respectively [34, 35]. In studies of colorectal cancer and liver cancer, the level of TCA was associated with an increased risk of development of both cancer types [34, 36]. In the liver, TCA leads to upregulation of Toll-like receptor four signaling and an increase in inflammation and stellate cell activation [36].
Despite the novelty of this study, a few caveats must be noted. The study assessed the SI microbiome by testing duodenal biopsy samples, resulting in microbiome profiles that are more reflective of the mucosa-associated microbiome rather than the luminal microbiome. However, given the importance of mucosal adherent bacteria in gut permeability, we believe that the mucosal microbiome has pathophysiological relevance to downstream effects in the liver. Additionally, the duodenal microbiome may not be completely representative of the entire microbiome of the SI. While there are no human studies comparing duodenal samples to jejunal or ileal samples, mouse studies have shown that duodenal composition is similar in alpha diversity, beta diversity, and bacterial composition as the rest of the SI [37]. As this is a relatively young cohort, we observed a low number of incident HCC diagnoses. Based on longitudinal power calculation, 227 patients with 14 incident events had a power to detect large differences (i.e., hazard ratios of 7.68 or higher). However, despite the low number of participants with incident HCC, we were able to identify several metabolites and microbial biomarkers that were significantly associated with HCC development after adjusting for false discovery. But we are aware that the more subtle markers may have been missed as the study was underpowered to capture small differences. Validation of these findings will be needed in an independent cohort.
Conclusion
This study shows for the first time the association of the small intestinal microbiome in the development of HCC. The associations of methionine, TCA, and Alloprevotella with gut barrier function, inflammation, and carcinogenesis support the hypothesis that these biomarkers may be markers of pathogenic processes that contribute to HCC development and could help to inform new HCC prevention or treatment strategies. We propose that microbial sampling through duodenal biopsy at the time of variceal screening via an EGD can provide significant information for biomarker development and possible prognostication for patients with cirrhosis.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplemental Figure 1: Principal component analysis (PCA) plot of serum metabolites comparing patients that did develop HCC versus those that did not (p.adj=0.10). P-value was computed using adonis and adjusted for age, sex, and hepatic encephalopathy. (TIFF 1048 kb)
Author’s contribution
TSD, JPJ, SKH conceived the design of the study, performed data collection, data analysis, and interpretation, and was involved in drafting the manuscript with revisions. SKH provided funding. VA, JRP, FD, PE, VS, JNB, MZ, VL, GC, WA, OF, and MG provided crucial research components and was involved in the editing and revisions of the manuscript. DE provided ideas on experimental design, data analysis, and interpretation, and edits to the manuscript.
Funding
This work was supported by a Grant from the National Institutes of Health/National Cancer Institute (R01CA204145). T.S.D. was supported by NIH T32 DK 07180 and J.P.J. was supported by VA Career Development Award IK2CX001717.
Declarations
Conflict of interest
All authors do not have any potential conflicts of interest.
Footnotes
Guarantor of the article: Shehnaz K. Hussain accept full responsibility for the conduct of the study and has access to the data and have control of the decision to publish.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 2.Howlader N, Noone A, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2014. National Cancer Institute. 2018:posted to the SEER web site, April 2017.
- 3.Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet (Lond Engl) 2003;362:1907–1917. doi: 10.1016/S0140-6736(03)14964-1. [DOI] [PubMed] [Google Scholar]
- 4.Singal AG, Pillai A, Tiro J. Early detection, curative treatment, and survival rates for hepatocellular carcinoma surveillance in patients with cirrhosis: a meta-analysis. PLoS Med. 2014;11:e1001624. doi: 10.1371/journal.pmed.1001624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Albhaisi S, Shamsaddini A, Fagan A. Gut microbial signature of hepatocellular cancer in men with cirrhosis. Liver Transpl. 2021;27:629–640. doi: 10.1002/lt.25994. [DOI] [PubMed] [Google Scholar]
- 6.Yu LX, Schwabe RF. The gut microbiome and liver cancer: mechanisms and clinical translation. Nat Rev Gastroenterol Hepatol. 2017;14(9):527–539. doi: 10.1038/nrgastro.2017.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu LX, Yan HX, Liu Q, et al. Endotoxin accumulation prevents carcinogen-induced apoptosis and promotes liver tumorigenesis in rodents. Hepatology. 2010;52(4):1322–1333. doi: 10.1002/hep.23845. [DOI] [PubMed] [Google Scholar]
- 8.Zoetendal EG, Raes J, van den Bogert B, et al. The human small intestinal microbiota is driven by rapid uptake and conversion of simple carbohydrates. ISME J. 2012;6:1415–1426. doi: 10.1038/ismej.2011.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martinez-Augustin O, Sanchez de Medina F. Intestinal bile acid physiology and pathophysiology. World J Gastroenterol. 2008;14:5630–5640. doi: 10.3748/wjg.14.5630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Romero-Gomez M, Jover M, Galan JJ, Ruiz A. Gut ammonia production and its modulation. Metab Brain Dis. 2009;24:147–157. doi: 10.1007/s11011-008-9124-3. [DOI] [PubMed] [Google Scholar]
- 11.Angelakis E, Armougom F, Carrière F, et al. A metagenomic investigation of the duodenal microbiota reveals links with obesity. PLoS ONE. 2015;10:e0137784. doi: 10.1371/journal.pone.0137784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raja G, Jung Y, Jung SH, Kim T-J. 1H-NMR-based metabolomics for cancer targeting and metabolic engineering—a review. Process Biochem. 2020;99:112–122. doi: 10.1016/j.procbio.2020.08.023. [DOI] [Google Scholar]
- 13.Raja G, Gupta H, Gebru YA, et al. Recent advances of microbiome-associated metabolomics profiling in liver disease: Principles, mechanisms, and applications. Int J Mol Sci 2021;22. [DOI] [PMC free article] [PubMed]
- 14.Schwabe RF, Greten TF. Gut microbiome in HCC—mechanisms, diagnosis and therapy. J Hepatol. 2020;72(2):230–238. doi: 10.1016/j.jhep.2019.08.016. [DOI] [PubMed] [Google Scholar]
- 15.Yu L-X, Schwabe RF. The gut microbiome and liver cancer: mechanisms and clinical translation. Nat Rev Gastroenterol Hepatol. 2017;14:527–539. doi: 10.1038/nrgastro.2017.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martino C, Morton JT, Marotz CA, et al. A novel sparse compositional technique reveals microbial perturbations. mSystems. 2019;4:e00016-19. doi: 10.1128/mSystems.00016-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lozupone CA, Knight R. Species divergence and the measurement of microbial diversity. FEMS Microbiol Rev. 2008;32:557–578. doi: 10.1111/j.1574-6976.2008.00111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15(12):550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Storey JD, Tibshirani R. Statistical significance for genomewide studies. Proc Natl Acad Sci U S A. 2003;100(16):9440–9445. doi: 10.1073/pnas.1530509100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J R Stat Soc Ser B (Methodol) 1995;57(1):289–300. [Google Scholar]
- 21.Koh H, Livanos AE, Blaser MJ, Li H. A highly adaptive microbiome-based association test for survival traits. BMC Genom. 2018;19(1):210. doi: 10.1186/s12864-018-4599-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Keng VW, Largaespada DA, Villanueva A. Why men are at higher risk for hepatocellular carcinoma? J Hepatol. 2012;57(2):453–454. doi: 10.1016/j.jhep.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132(7):2557–2576. doi: 10.1053/j.gastro.2007.04.061. [DOI] [PubMed] [Google Scholar]
- 24.Chen CL, Kuo MJ, Yen AM, et al. Gender difference in the association between metabolic factors and hepatocellular carcinoma. JNCI Cancer Spectr. 2020;4(5):pkaa036. doi: 10.1093/jncics/pkaa036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu Q, Li F, Zhuang Y, et al. Alteration in gut microbiota associated with hepatitis B and non-hepatitis virus related hepatocellular carcinoma. Gut Pathog. 2019;11:1. doi: 10.1186/s13099-018-0281-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Downes J, Dewhirst FE, Tanner ACR, Wade WG. Description of Alloprevotella rava gen. nov., sp. nov., isolated from the human oral cavity, and reclassification of Prevotella tannerae Moore et al. 1994 as Alloprevotella tannerae gen. nov., comb. nov. Int J Syst Evol Microbiol. 2013;63:1214–1218. doi: 10.1099/ijs.0.041376-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cavuoto P, Fenech MF. A review of methionine dependency and the role of methionine restriction in cancer growth control and life-span extension. Cancer Treat Rev. 2012;38(6):726–736. doi: 10.1016/j.ctrv.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 28.Guo H, Lishko VK, Herrera H, Groce A, Kubota T, Hoffman RM. Therapeutic tumor-specific cell cycle block induced by methionine starvation in vivo. Cancer Res. 1993;53(23):5676–5679. [PubMed] [Google Scholar]
- 29.Pascale RM, Peitta G, Simile MM, Feo F. Alterations of methionine metabolism as potential targets for the prevention and therapy of hepatocellular carcinoma. Medicina (Kaunas) 2019;55:296. doi: 10.3390/medicina55060296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Raja G, Cao S, Kim D-H, Kim T-J. Mechanoregulation of titanium dioxide nanoparticles in cancer therapy. Mater Sci Eng C. 2020;107:110303. doi: 10.1016/j.msec.2019.110303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mahmood N, Cheishvili D, Arakelian A, et al. Methyl donor S-adenosylmethionine (SAM) supplementation attenuates breast cancer growth, invasion, and metastasis in vivo; therapeutic and chemopreventive applications. Oncotarget. 2018;9(4):5169–5183. doi: 10.18632/oncotarget.23704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang W, Xiang YB, Li HL, et al. Vegetable-based dietary pattern and liver cancer risk: results from the Shanghai women's and men's health studies. Cancer Sci. 2013;104(10):1353–1361. doi: 10.1111/cas.12231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang X, Fu X, Van Ness C, Meng Z, Ma X, Huang W. Bile Acid Receptors and Liver Cancer. Curr Pathobiol Rep. 2013;1(1):29–35. doi: 10.1007/s40139-012-0003-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ridlon JM, Wolf PG, Gaskins HR. Taurocholic acid metabolism by gut microbes and colon cancer. Gut Microbes. 2016;7(3):201–215. doi: 10.1080/19490976.2016.1150414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wolf PG, Gaskins HR, Ridlon JM, et al. Effects of taurocholic acid metabolism by gut bacteria: A controlled feeding trial in adult African American subjects at elevated risk for colorectal cancer. Contemp Clin Trials Commun. 2020;19:100611. doi: 10.1016/j.conctc.2020.100611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu Z, Zhang Z, Huang M, et al. Taurocholic acid is an active promoting factor, not just a biomarker of progression of liver cirrhosis: evidence from a human metabolomic study and in vitro experiments. BMC Gastroenterol. 2018;18:112. doi: 10.1186/s12876-018-0842-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gu S, Chen D, Zhang JN, et al. Bacterial community mapping of the mouse gastrointestinal tract. PLoS ONE. 2013;8(10):e74957. doi: 10.1371/journal.pone.0074957. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: Principal component analysis (PCA) plot of serum metabolites comparing patients that did develop HCC versus those that did not (p.adj=0.10). P-value was computed using adonis and adjusted for age, sex, and hepatic encephalopathy. (TIFF 1048 kb)