Abstract
Left–right asymmetries in the nervous system (lateralisation) influence a broad range of behaviours, from social responses to navigation and language. The role and pathways of endogenous and environmental mechanisms in the ontogeny of lateralisation remains to be established. The domestic chick is a model of both endogenous and experience-induced lateralisation driven by light exposure. Following the endogenous rightward rotation of the embryo, the asymmetrical position in the egg results in a greater exposure of the right eye to environmental light. To identify the genetic pathways activated by asymmetric light stimulation, and their time course, we exposed embryos to different light regimes: darkness, 6 h of light and 24 h of light. We used RNA-seq to compare gene expression in the right and left retinas and telencephalon. We detected differential gene expression in right vs left retina after 6 h of light exposure. This difference was absent in the darkness condition and had already disappeared by 24 h of light exposure, suggesting that light-induced activation is a self-terminating phenomenon. This transient effect of light exposure was associated with a downregulation of the sensitive-period mediator gene DIO2 (iodothyronine deiodinase 2) in the right retina. No differences between genes expressed in the right vs. left telencephalon were detected. Gene networks associated with lateralisation were connected to vascularisation, cell motility, and the extracellular matrix. Interestingly, we know that the extracellular matrix—including the differentially expressed PDGFRB gene—is involved in morphogenesis, sensitive periods, and in the endogenous chiral mechanism of primary cilia, that drives lateralisation. Our data show a similarity between endogenous and experience-driven lateralisation, identifying functional gene networks that affect lateralisation in a specific time window.
Subject terms: Developmental biology, Neuroscience, Cognitive neuroscience
Introduction
Left–right asymmetries (lateralisation) are a major principle of organization of the nervous system in both vertebrate and invertebrate species1–4. Anatomical and functional lateralisation has a central role in behaviour and cognition from foraging to navigation, limb use, attention, communication, social responses, and language1,3–6. Lateralisation often provides increased neural and cognitive efficiency1,3,7–12, whereas atypical lateralisation is associated with disease13,14. Despite the importance of lateralisation, its ontogenesis is far from being understood. Endogenous, genetically-guided mechanisms drive the emergence of internal organ asymmetries15. Endogenous mechanisms, though, do not explain the whole ontogenesis of lateralisation11,16,17, or the dissociation of lateralisation patterns observed across different tissues6,18,19. It has been suggested that genetic factors explain only a small fraction of asymmetries in human handedness20. Here we focus on the ontogeny of experience-driven lateralisation and its connection to endogenous lateralisation, using domestic chicks as a model system.
Endogenous mechanisms have been identified as factors that initiate systematic left–right asymmetries21,22. In particular, mounting evidence shows that left–right asymmetry in embryonic development is mediated by cilia23. Cilia are dynamic, hair-like cell organelles that extend from the cell surface into the extracellular space13,14,24,25. They play an important role in detecting changes in the extracellular environment and transmitting signals to the cell that regulate developmental and physiological processes, including sensory function, cell adhesion and establishing the left–right body axis24,26–29. Due to molecular chirality, motor cilia induce a leftward flow of extracellular fluid detected by primary (or sensory) cilia30. These activate stronger left-sided Ca2+ signalling31, stronger left-sided expression of the Nodal pathway13, reviewed in Ref.14, and in turn produce left–right asymmetries. Defects of cilia include the lateralised situs inversus phenotype, where the major visceral organs appear flipped compared to the typical position32. While the role of cilia in the asymmetries of the central nervous system is not entire clear, more than a hundred human disorders have been linked to defects in cilia33. In the chick, the endogenous left–right asymmetry is established through an alternative mechanism, based on the asymmetric cell displacement of cells expressing sonic hedgehog (SHH) and fibroblast growth factor 8 (FGF8) in Hensen’s node34.
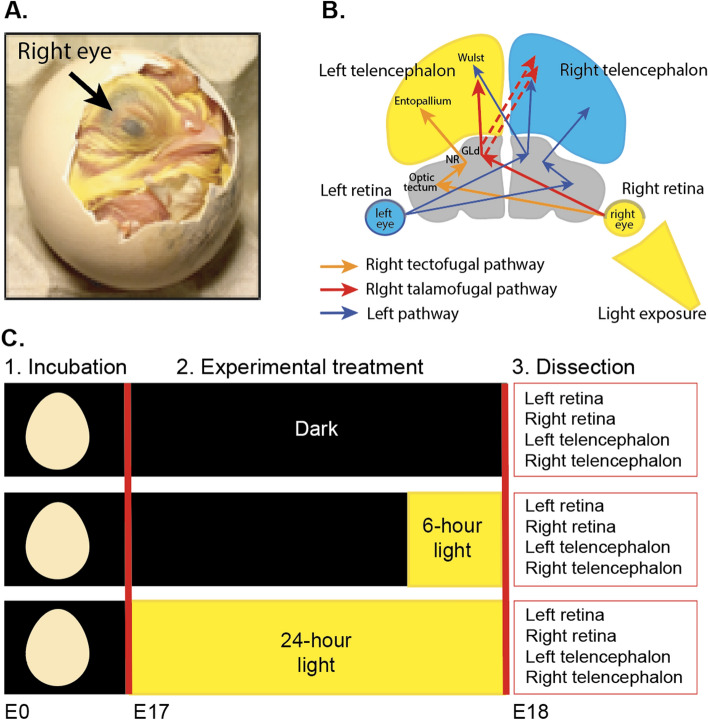
Beside endogenous mechanisms, environmental/experience-related factors35,36 and epigenetic factors36–38 affect lateralisation at the anatomical and functional levels. The role of experience-related factors in the ontogenesis of lateralisation is apparent in different taxa11,39–44. In fish and birds11,37,40,45–47, embryonic light stimulation triggers anatomical and functional lateralisation building on pre-existing endogenous-driven asymmetries. In birds, the rightward torsion of the embryo47–49 asymmetrically exposes the right eye to the light that filters through the egg, while the left eye is covered by the body (see Fig. 1A). This asymmetrical light exposure induces both anatomical and functional lateralisation19,49–54, although some brain asymmetries do not depend on light18. In light-incubated chick embryos, light reaches the right retina (first stage of the input), that feeds the telencephalon (see Fig. 1B) via the tectofugal and thalamofugal pathways (see orange and red arrows in Fig. 1B for the right-fed pathway). We analysed the effect of light and its effect at different times on the retina and telencephalon, which are the initial and last stations of the visual system affected by light exposure.
Figure 1.
(A) Asymmetric position of chick embryos. By embryonic day 17 (E17), the right eye is located toward the shell and the left eye tucked under the wing. Only right eye is directly exposed to light. (B) Representation of the visual system in chicks. The two arrows that depart from the retina indicate the tectofugal and thalamofugal pathways (see55). The left tectofugal pathway (orange pathway via right retina, left optic tectum, left nucleus rotundus (NR) and left entopallium in the left telencephalon) and left thalamofugal pathway (red pathway via right retina, left thalamic nucleus geniculatus lateralis pars dorsalis (GLd), left Wulst in the left telencephalon and right Wulst in the right telencephalon) are shown. The right tectofugal and thalamofugal pathways (fed by the left retina) are represented in blue. The regions used in the RNA-seq analysis (retina and telencephalon) are coloured. (C) Schematic representation of the experimental phases. Eggs were incubated in the dark from embryonic day 0 (E0) to embryonic day 17 (E17). On day E17, eggs were randomly assigned to one of three different experimental treatments. One group continued incubation in darkness (Dark), one group was exposed to 6 h of light (6-h light) and one group was exposed to 24 h of light (24-h light). At the end of the experimental treatment (E18), dissections were conducted. After dissection, sex was determined by PCR. RNA-seq analysis was carried out on samples from 5 males per light regime.
We focused on the domestic chick (Gallus gallus), a prominent model to investigate light-induced lateralisation45,49,52,55–57. This model has several advantages: chicks are classic models in embryology and developmental biology, with many details known about the cellular and molecular pathways that mediate general development, visual development and asymmetry formation58,59, the ease of manipulating light exposure during egg incubation and post-hatch39,55, the almost complete decussation of the fibres of the optic nerve and reduced number of connections between the two brain hemispheres present in birds51, and the ease of testing a precocial species that has a mature motor and sensory system at the beginning of life60.
The effect of embryonic light exposure on lateralisation is apparent at specific time windows19,39,44,45,49,55. In chicks, two hours of light exposure in the last 3 days of incubation (E17-E20 according to Ref.61) are sufficient to determine light-induced behavioural lateralisation, while 6 h of light exposure make this lateralisation irreversible by the temporary occlusion of the right eye with an eye patch39. This transient time window for the induction of environmental-driven lateralisation exhibits the distinctive features of sensitive periods39,62. Sensitive periods are specific times during development, in which specific experience-dependent events tune connectivity patterns within the functional range63,64. The closure of sensitive periods of brain plasticity can be self-terminated by experience, including exposure to light and visual stimuli, as is clear for filial imprinting in chicks62,65. The closure of sensitive periods and neural plasticity are regulated by molecular events that halt/enhance neural plasticity. Many molecular factors that initiate and close plasticity in sensitive periods, from neuromodulatory signals to synaptic proteins and components of the extracellular matrix, are influenced by visual experience (reviewed in Ref.63). One example is the control of thyroid hormone signalling, mediated by the type-2 deiodinase/iodothyronine deiodinase 2 (DIO2)66 (see67 for chicks), that is involved in retinal development68. Molecules associated with the sensitive periods that regulate the effect of light on lateralisation have not been identified yet.
A broader issue about the ontogenesis of lateralisation is whether environmental exposure to light acts through the same pathway of endogenous asymmetrical mechanisms that produce embryonic rotation and bending towards the right in vertebrate species69–71, or via a separate, dedicated mechanism. To shed light on the molecular pathways involved in light-driven lateralisation in chicks, we investigated the effect of light exposure on gene expression in the right and left retinas and telencephalon through an RNA-seq analysis. We exposed E17 embryos to three experimental conditions: complete darkness (Dark), 5–8 h light exposure (6-h light), 24–27 h of light exposure (24-h light) (Fig. 2A). By exposing embryos to at least 5 h of light, we targeted responses subsequent to those of immediate early genes72–74 and reached the amount of stimulation (between 2.5 and 6 h of light exposure) that provides irreversible lateralisation in chicks39. We dissected chicks at E18 (around developmental Stage 4461), before processes related to hatching started. With this design, we looked at the short term and sustained effect of embryonic light exposure in the retina and telencephalon compared to no light exposure.
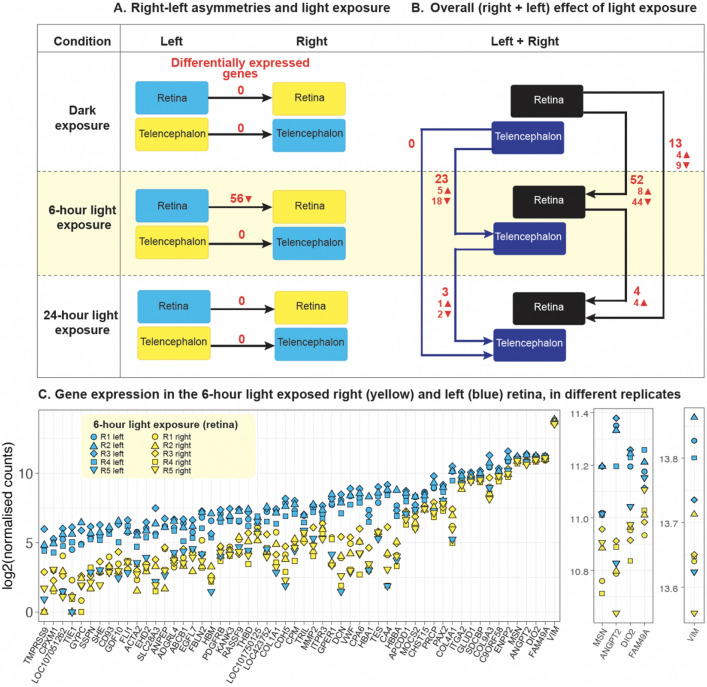
Figure 2.
(A) Diagram of the left–right comparisons and differentially expressed genes (in red) for each comparison (filled triangle—upregulation, inverted filled triangle—downregulation). (B) Diagram of the overall (right + left) effect of different exposure to light (darkness, 6-h, 24-h) as differentially expressed genes (in red) in the retina and telencephalon. (C) Gene expression. For each gene differentially expressed in the retina at 6-h light exposure, the log2(normalised counts) are shown on the y axis. A supplementary close-up is presented on the right panels for better visualisation of all replicates. Yellow symbols indicate the replicates of the right retina, blue symbols indicate replicates of the left retina. Different symbols indicate different replicas, as shown in the legend.
Materials and methods
Samples and study design
Sixty-three freshly fertilized eggs of Ross 308 (Aviagen) strain were stored in darkness at 4 °C until the beginning of incubation. Having 21 eggs in each experimental group provided us with enough embryos to dissect, despite the initially unknown number of unfertilised eggs and sex composition of the sample. Eggs were incubated at a constant temperature of 37.7 °C and 40% humidity and rotated automatically in complete darkness from embryonic day 0 (E0) to embryonic day 17 (E17) at 9:00 am. Subsequently, eggs were randomly assigned to experimental groups: in the Dark condition eggs continued to stay in darkness, eggs in the 24-h light exposure condition received light from E17 9:00 am to ~ E18 12:00 pm (i.e., 24–27 consecutive hours of light), and eggs in the 6-h light exposure condition received light from E18 4:00 am to ~ E18 12:00 pm (i.e., 5–8 consecutive hours of light) (Fig. 1C). This timing of exposure and dissection was chosen to make sure light exposure was long enough to induce functional lateralisation55 and at the same time target responses subsequent to those of immediate early genes72–74. Light was delivered to eggs through five white-light LEDs (18 lumens per LED) placed at 24 cm from the eggs. We conducted 44 dissections on E18 between 9 am-12 pm (unfertilised and surplus eggs were not dissected): 14 in the dark condition, 15 in the 6-h exposure condition, 15 in the 24-h exposure condition. After molecular sexing and quality control analyses on RNA samples, we chose 5 male samples for each light regime, based on the best quality control indexes. After quality control analyses on the RNA samples, we randomly choose 5 male samples for each of the light regimes based on the best quality control indexes.
Dissection
A total of 44 embryos were dissected. Experiments with bird embryos did not require specific protocol approval when these studies were carried out (i.e., 2016), as per the Italian and European Community laws for the ethical treatment of animals (Legislative decree law 26/2014). Our reporting follows ARRIVE guidelines. For each embryo, the egg was retrieved from the incubator, placed at − 20 °C for 2 min and then immediately opened by cutting along the widest part of the shell with scissors. Embryonic membranes were then disrupted and the embryo was culled by rapid decapitation followed by the removal of both eyes and then brain. We included embryos that macroscopically corresponded to developmental Stage 44 and took notes of potential differences61. The dissection of retinas and telencephalons were performed in parallel by two experimenters to minimize sample collection time. Brain tissue was macroscopically subdivided in two regions: telencephalon and remainder of the brain. Of these regions, only telencephalon was further subdivided into left and right samples and processed for RNA-seq. Macrodissected brain samples were frozen immediately on dry ice and stored at − 80 °C until RNA extraction. For retina dissection, first, eyelids were cut away and the whole eye was carefully removed without piercing the eye globe. Then, dissection of the retina from the enucleated eyes was performed by cutting the eyes in half to expose the vitreous and retina. The translucent gelatinous vitreous humour was carefully removed with forceps exposing the retina. Retina and pigmented epithelium were removed from the eye cup and placed immediately on dry ice and stored at − 80 °C until RNA extraction.
Molecular sexing
Since anatomic asymmetries in the thalamofugal pathway75 and behavioural asymmetries76,77 are more pronounced in male chicks compared to females (reviewed in Ref.78), we focused our study on males only. Sexing was performed according to Ref.79.
RNA extraction and quality control
Total RNA was extracted and DNAse-treated using RNeasy mini kit (Qiagen) with DNAse treatment, following the manufacturer’s protocol. RNA concentrations were measured using the Qubit RNA Broad-Range Assay kit and RNA quality was analysed on an Agilent Bioanalyzer 2100. Only samples with RNA integrity numbers (RIN) ≥ 9 were considered for RNA-seq inclusion.
RNA-seq analysis
RNA-seq data were generated from a total of 60 samples (5 embryos × 3 experimental groups × 2 tissues × 2 hemispheres) on an Illumina HiSeq 4000 at the Genomic facilities of the Wellcome Trust Human Genetic Centre (WTHGC), University of Oxford. Five biological replicates were used for each tissue (telencephalon and retina), side (left and right) and condition (dark, 6-h and 24-h light exposure). One µg of input RNA was used for the standard TruSeq Stranded mRNA Library Preparation Kit that includes isolation of mRNA by polyA-selection. The libraries were then pooled according to their tissue, side and treatment group. The sequence libraries had a median number of 11,232,013 reads before trimming (10,261,901 reads after trimming).
All data were checked for quality with FastQC (FastQC v0.11.5, 2017 https://www.bioinformatics.babraham.ac.uk/projects/fastqc/) and contaminants with FastQ Screen (v0.10.0), then trimmed for adapter sequences with Trimmomatic (v0.36)80. The statistics of number of reads per sample are shown in Supplementary Table 4.
Differential gene expression analysis
Surviving reads were quantified by mapping against the Ensembl galGal6 chicken transcriptome (downloaded from ftp://ftp.ncbi.nlm.nih.gov/genomes/all/GCF/000/002/315/GCF_000002315.6_GRCg6a/GCF_000002315.6_GRCg6a_rna.fna.gz) using Salmon (v0.12.0)81. Mapping efficiency ranged from 76 to 81%. Transcript read counts were collapsed to genes with tximport82 in R version 4.0.3 (https://www.R-project/org), then counts and fragment lengths were imported into DESeq2 (1.30.0)83. Principal components analysis (PCA) was applied to visualize variance in the data, and one individual in the 24-h light exposure treatment group was excluded from further analysis because it was an outlier and it was annotated as underdeveloped at the time of dissection. Sequencing replicates of each sample library were similar by PCA, so they were combined to increase statistical power. Differential gene expression analysis was conducted separately for retina and telencephalon tissues. With DESeq2, we compared gene expression in left and right sides of the same tissue in the same treatment conditions, adding a factor to our model to control for individual effects, and we also looked for aggregate differences in gene expression between treatment groups (reference code in Available data for details). An adjusted p-value cut-off of p < 0.05 was applied for all comparisons. Analysis was conducted with R (https://www.R-project.org/) and RStudio (http://www.rstudio.com). The packages dplyR (https://CRAN.R-project.org/package=dplyr), biomaRt84, tximport82 and independent filtering was employed to optimize detection of genes below this threshold. Shrinkage estimation was applied to moderate fold-changes of low abundance genes to facilitate ranking for downstream analysis of gene ontology.
Results
Differential gene expression: lateralised and overall effects
To identify genes differentially expressed in the right and left hemisphere in the absence or presence of asymmetric light exposure, we compared left vs right gene expression in both tissues (retina and telencephalon) in the Dark, 6-h light and 24-h light conditions, using a within-subjects design (Fig. 2A). In the retina, we identified 56 differentially expressed (DE) genes (adjusted p-value < 0.05, Supplementary Table 1) in the right vs left side after 6 h of light exposure (Fig. 2C). All differentially expressed genes were downregulated in the right vs. left retina (see also Ref.85). Besides genes involved in the circulatory system development and extracellular matrix/cell motility (see “Gene set enrichment analysis” section below) we identified two differentially expressed genes of interest. The first, DIO2, is involved in sensitive periods66,67 and in the development of the retina in the chicken and other vertebrates68. The second, PAX2, is a transcription factor that plays an important role in the glia during retinal development in the chicken embryo86 and in eye development in vertebrates87. We found no significant left–right differences in gene expression in either darkness or after 24 h of light exposure in the retina. No significant differences in gene expression were identified in the telencephalon.
To identify the overall, non-lateralised effect of light exposure, we compared gene expression of both brain sides (i.e., right plus left retina, right plus left telencephalon) between different light exposure conditions (Dark vs 6-h light, Dark vs 24-h light, 6-h light vs 24-h light) (Fig. 2B). In both tissues, the largest effect has been found in the Dark vs 6-h light exposure comparison (52 differentially expressed (DE) genes in the retina, 23 in the telencephalon). In the 6-h vs 24-h comparison we found 4 DE genes in the retina, 3 in the telencephalon; in the dark vs 24-h exposure comparison we found 13 DE genes in the retina (4 upregulated, 9 downregulated, including CAPN15, that is involved in the development of the visual system) and 0 in the telencephalon (see Supplementary Table 2A–E). The reduction of DE genes after more than 6 h of light exposure corroborates behavioural and neuroanatomic evidence that, in different species, embryonic light exposure has a transient effect (e.g. see45 for different time windows in chicks, see46 for zebrafish). Behavioural evidence suggests that, in domestic chick embryos (E19), this self-terminating sensitive period ends after 6 h of light exposure, during the last three days of incubation39.
Gene set enrichment analysis
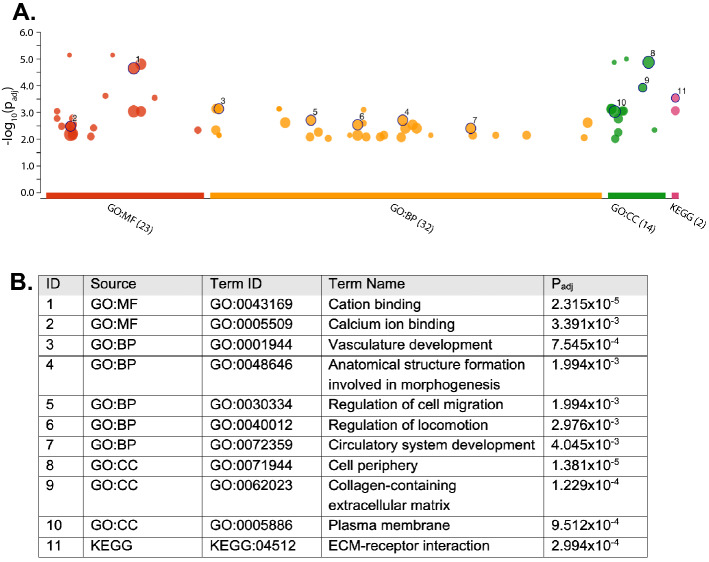
To identify biological pathways connected to significant asymmetrical light exposure, we used the differentially expressed genes identified above (Retina dark vs 6-h exposure) and performed a gene enrichment analysis via g:Profiler88,89, with 16,356 protein-coding genes used in the model as the background, and FDR at 1%. In the Retina dark vs 6-h exposure comparison, 32 biological process GO terms, 14 cellular component terms and 23 molecular function GO terms were enriched (Fig. 3A). A partial list of enriched terms is shown in Fig. 3B (for the complete list see Supplementary Table 3).
Figure 3.
Gene enrichment analysis: functional terms and KEGG pathways. (A) g:Profiler Manhattan plot. The x axis shows the functional terms (term size greater than 50), grouped and colour-coded by data source (red = GO molecular function, orange = GO biological process, green = GO cellular component, pink = KEGG database). The position of terms in the plots is fixed and terms from the same gene ontology branch are close to each other. The size of circles shows the corresponding term size. Numbers correspond to the terms in the table below; the full list of terms is available in Supplementary Table 3. (B) Legend gene enrichment analysis. The table shows the ID referred to the Manhattan plot above, source database, term ID, term name, adjusted p values after FDR correction. See Supplementary Table 3 for the full list of terms.
The enriched biological processes include two systems, namely the development of the vascular/circulatory system and the formation of the anatomical structure, which highlight an involvement of ontogenetic plasticity. The enriched biological processes relate to two main systems: vascular/circulatory system development and anatomical structure formation. In line with these results, in studying the functional genetics of handedness and lateralisation, Schmitz et al.38 found enrichment on GO terms related to anatomic structure development, including cardiovascular system development, artery development and epithelial tube morphogenesis. The enriched cellular components include the extracellular matrix (that provides physical scaffolding for the cellular constituents and can initiate biochemical and biomechanical cues required for tissue morphogenesis, differentiation and homeostasis), plasma membrane (GO:0005886), haptoglobin-haemoglobin complex (GO:0031838), cell periphery (GO:0071944) and haemoglobin complex (GO:0005833) as top hits. Finally, the enriched molecular functions include metal ion binding (GO:0046872), cation binding (GO:0043169) and platelet-derived growth factor binding (GO:0048407), haptoglobin binding (GO:0031720) and oxygen carrier activity (GO:0005344) and metallocarboxypeptidase activity (GO:0004181) as top hits. Using the same approach, no functional enrichment was found in other tissues and conditions.
KEGG90 pathway analysis confirmed the significant enrichments for extracellular matrix (ECM) receptor-interaction, that is important in tissue morphogenesis and in the maintenance of tissue and function, and focal adhesion, that is involved in cell motility proliferation and differentiation. Interestingly, the membrane of primary cilia has been associated with receptors for ECM proteins26,91. Similarly, in studying the functional genetics involved in handedness with KEGG pathway analysis, Schmitz et al. found enrichment for ECM-receptor interaction and focal adhesion. Moreover, they found enrichment in the TGF-beta signalling pathway. This pathway is connected to PDGFRB (platelet-derived growth factor receptor β), which we found to be differentially expressed in right vs left retina at 6 h of light exposure. This gene, essential for the development of the cardiovascular system, helps in the rearrangement of the actin cytoskeleton and is involved in the dynamic primary cilia mechanism24,92. This further evidence points toward the implication of primary cilia in the experience-driven, light-triggered gene pathway of lateralisation initiated in the eye13.
Overall, gene set enrichment analysis shows an involvement of blood vessels/circulatory system development and processes related to morphogenesis in response to 6 h of light exposure in the retina. Moreover, we detected a temporal-specific effect in the retina present after 6 h of light stimulation but not after 24 h. No enrichment was present in other experimental conditions (bilateral tissue sampled at different light-exposure times) and in the telencephalon.
Interaction network
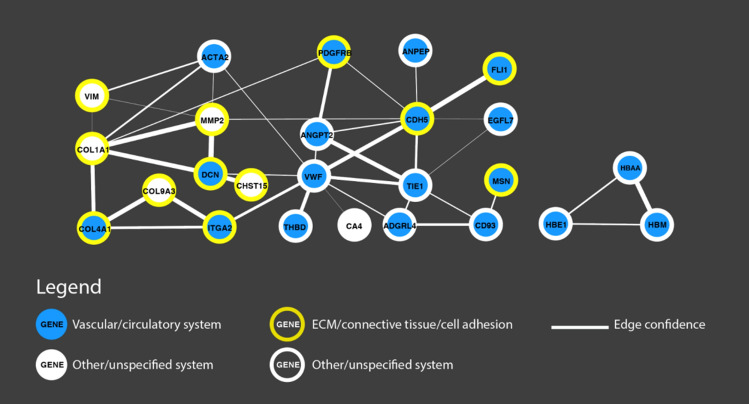
To investigate the protein interaction network of the differentially regulated genes involved with embryonic light exposure (left–right asymmetry after 6-h light exposure), we analysed the differentially expressed genes using STRING v11.093 and visualised its output with Cytoscape94,95. We analysed the full network (physical and non-physical interactions) with all available interaction sources and medium confidence cut-off (required interaction score ≥ 0.4). Of the 56 differentially expressed genes for Retina 6-h light exposure, 53 could be found in STRING. The 53 nodes in the overall network produce 40 interactions (vs 6 expected interactions if the nodes were selected at random), with a significant protein–protein interaction enrichment (p < 1.0e−16), involving 25 genes. Two interaction subnetworks have been identified (see Fig. 4).
Figure 4.
Interaction network of genes differentially expressed in the right vs left retina in the 6-h light exposure condition. Physical and non-physical interactions are represented, based on all available sources in Cytoscape94 (text mining, experiments, databases, co-expression, neighbours, gene fusion and co-occurrence). Involved systems and functions (e.g., vascular/circulatory system, extracellular matrix (ECM)/connective tissue/cell adhesion are coloured according to the legend, larger edges indicate stronger confidence in the interaction.
The main network (left in Fig. 4) includes nodes involved in the vascular/circulatory system (blue circle) and in connective tissue/cell adhesion/extracellular matrix (yellow outline); the haemoglobin-complex subnetwork (right) is linked to the vascular/circulatory system.
Discussion
The mechanisms that drive the ontogenesis of lateralisation are far from being understood. Due to ease of egg manipulation and brain architecture, birds are prominent models to investigate the role of environmental factors in lateralisation11 and eye development59. We used chicken embryos to identify genetic pathways involved in lateralisation induced by asymmetrical light stimulation. We compared gene expression in the right and left retinas and telencephalon in embryos exposed to different light regimes. In natural settings, light exposure is regulated by the parents, that systematically interrupt incubation in the last days before hatching, as shown in pigeons and chickens96,97. In these periods, light reaches the egg and embryo, enhancing ontogenetic plasticity.
After exposing embryos to darkness, 6-h light or 24-h light, we dissected samples at the same developmental stage (embryonic day E18) and compared the effects of endogenous and stimulus-driven lateralisation induced by light in the right and left brain. We identified significantly differentially expressed genes in the retina in the 6-h light exposure condition (Fig. 2A). Because all samples have been collected at the same developmental stage, we conclude that the observed differential gene expression between left and right retina is due to light exposure.
All differentially expressed genes were downregulated in the right vs left retina, suggesting a potential inhibitory effect of light during ontogenesis. In line with this scenario, Manns et al.85 have previously shown that embryonic light stimulation in pigeon induces asymmetric inhibition of TrkB ⁄Ras activity (related to morphogenesis) in the tectum fed by the right eye. These cellular asymmetries emerge in the tectum only after hatching. A downregulation in the extracellular matrix (ECM), with consequent inhibition of plasticity98, is consistent with an inhibitory effect of light. In the present work, we exposed embryos to at least 6 h of light, thus avoiding the signature of peak expression of immediate early genes. Future work should investigate early effect of light exposure (before 6 h of exposure) on neurons and ECM, as well as the connection between retinal light exposure and inhibition in other brain areas.
Our findings support the idea that differential gene expression induced by light exposure terminates between 6 and 24 h of light exposure in the retina. The lack of differences observed after 24 h of light exposure, while differences are detected at 6 h of light exposure, confirms previous findings about the presence of transient sensitive periods in the development of the nervous system in chicks (for chicks see62,74,99), and other vertebrates66,100 (see101 for developmental changes in human foetuses). Moreover, the timing of this effect is in line with the fact that inversion of lateralisation in chicks has been achieved only between 2.5 and 6 h of light exposure at E19/E2039. Overall, this pattern of data suggests that, between 6 and 24 h of light exposure the sensitive period of neural plasticity that stabilises lateralisation in the retina self-terminates. This hypothesis is corroborated by the significant differential expression of DIO2 (iodothyronine deiodinase 2) in the 6-h light exposure but not in the 24-h light exposure. DIO2 is a thyroid hormone converting enzyme with an essential role in neurodevelopment and the regulation of the sensitive periods in mammals and birds66. DIO2 has a role in the development of the retina in different vertebrates, including the chicken, where is involved in spatiotemporally specified waves of cone differentiation68. In the chick retina, the expression of thyroid hormone regulators follows a series of spatiotemporal waves, moving from the central retina to the periphery. It has been suggested that DIO2 likely blocks/postpones photoreceptor differentiation, thus increasing photoreceptors in specific areas of the retina102. More in general, modulation of DIO2 can finely tune the availability of triiodothyronine (and hence the presence of thyroid hormones) at specific times during brain development. This gene regulates the start of the sensitive period for filial imprinting in chicks (the self-terminating mechanism that mediates affiliative learning), as well as the regulation of predisposed preferences in domestic chicks, such as the preference for biological motion67,103 and objects that move changing in speed. Evidence that light exposure has a transient effect in a specific time window comes also by the comparison of the samples from both sides of each tissue (right plus left retina, right plus left telencephalon). In these comparisons, in both tissues we identified more differentially expressed genes in the darkness vs 6-h light exposure than in the 6-h light exposure vs 24-h light exposure conditions (23 vs 3 DE genes in the telencephalon; 52 vs 4 DE genes in the retina, see Fig. 2B).
While the retina showed right vs left differential gene expression at 6 h of light exposure, we did not detect any difference between sides in the telencephalon. A similar lack of significant left–right differences in telencephalon has been previously reported in fish104 and in human foetuses in the perisylvian area (neocortex), an area with high interhemispheric asymmetry105, while only mild and age-dependent asymmetries have been recently found in human foetuses101).
The telencephalon is a large portion of the chicken pallium fed by both the thalamofugal and tectofugal pathways (see Fig. 1B. These brain areas are homologous to the geniculocortical and extrageniculocortical systems in mammals11). Lack of lateralised gene expression at E18 can hence be due to different factors. Since we dissected a large portion of the brain, the lack of differential expression could be due to more regionally restricted lateralised effects mediated by light. In addition, light exposure may have its maximum effect in the telencephalon more than 24 h after the beginning of light stimulation. Moreover, the stimulation of both tectofugal and thalamofugal pathways may activate both hemispheres, since the thalamofugal pathway has both ipsilateral and contralateral projections in the telencephalon, or some genes involved in ontogenetic development and plasticity might not have transcriptional modulation. Further experiments should clarify whether more anatomically restricted areas of the embryonic telencephalon are affected by light exposure and on what time course.
Gene enrichment analyses have revealed two main pathways associated with lateralised differences induced by light stimulation: the vascular/circulatory system and the extracellular matrix (ECM). An interesting overlap between gene networks involved in the vascular system and the ECM/connective tissue appears in the interaction network analysis (Fig. 4). It is worth discussing the connection between the vascular system, ECM and lateralisation in more detail. As for the vascular system, one of the first macroscopic asymmetries observed during vertebrate development is the right-side looping of the heart tube (see70 for chicks, reviewed in Ref.106). More in general, organogenesis is a highly asymmetric process and the biophysical mechanisms engaged in organogenesis involve the ECM. The ECM is engaged in organogenesis via proliferation and traction forces that are mediated for instance by the cytoskeleton (reviewed in Ref.107). The ECM plays an important role in controlling experience-dependent plasticity in the visual cortex during ontogenesis, acting on spine dynamics and axonal sprouting (as reviewed here Ref.98). This is confirmed by our results of significant differential expression in the ECM. Together with previous literature, our findings suggest a connection between the vascular system and extracellular matrix in establishing asymmetries. Overall, the involvement of blood vessels/vascular system and extracellular matrix in asymmetries support the hypothesis that genes associated with lateralisation might be involved in anatomic structure development guided by environmental cues, rather than in asymmetrical body formation per se (see also Ref.38).
The ECM is connected to ion activity and to the asymmetric movement of primary cilia, that drives asymmetries in mice and other vertebrate species23. Moreover, cilia are present in the retina at the level of photoreceptors and in other retinal cell types28,29, acting in the detection and transduction of visual signals, macromolecules and other signals108. At the molecular level, we found enrichment of ion activity, including Ca2+ ion binding, that is connected to the activity of cilia. Previous reports have highlighted the role of calcium ions in lateralisation (e.g., fish109, mice31, chicks110). The membrane of primary cilia includes receptors for ECM proteins26,91, and extensive signalling has been established between cilia and ECM, with defects in cilia associated with developmental defects24,26. Enrichment for ECM-receptor interaction and focal adhesion has been found in the functional genetics of handedness38, as well as enrichment in the TGF-beta signalling pathway. This pathway is connected to PDGFRB (platelet-derived growth factor receptor β), one of the differentially expressed genes in the 6-h light exposure left–right comparison. This gene, essential for the development of the cardiovascular system, helps in the rearrangement of the actin cytoskeleton and is involved in the primary cilia mechanism92. PDGFR β receptors are primarily located and activated outside the cilium, inducing disassembly of the cilium during morphogenesis. By controlling the assembly and disassembly of their primary cilium, cells can modulate the response to extracellular morphogenetic signals, and determine their cellular architecture (see dynamics in the corneal endothelium of mice111). Overall, our results suggest that there are similarities between the endogenous lateralisation mechanisms and environmentally driven lateralisation since in both cases there is an involvement of the extracellular matrix and the platelet-derived growth factor receptor PDGFRβ, that are usually connected to primary cilia.
Among the differentially expressed genes in the right vs left retina there is PAX2, a transcription factor that plays an important role in the glia during retinal development, as previously shown in the chicken embryo86 and in the development of the visual system in vertebrates87. PAX2 is involved in the generation of the optic stalk and in the first stage of chiasm asymmetric axonal migration87,112. PAX2 expression has also been reported in the chick retina at later developmental stages in Müller glial cells, astrocyte-like radial cells that can undergo reprogramming and repair in teleost fish and regenerate retinal neurons through a process of dedifferentiation and acquisition of multipotent progenitor identity113 (see also Ref.114 for a review). Our findings highlight genes that mediate the effect of environmental experience (light exposure) in the ontogeny of left–right asymmetries. The role of environmental factors in lateralisation is becoming progressively clearer4,11,17. Moreover, additive genetic factors explain only one quarter of the variance in human handedness, and it has been suggested that nonshared environmental factors can explain part of the remaining variance20,115 (but see Refs.116–118). Hence, to understand the ontogeny of functional and brain lateralization we must elucidate the interaction between endogenous and environmental factors2,3,11,14,17,66,67,69–71. It must also be considered how noncoding microRNA72, or epigenetic mechanisms, which affect gene activity and expression by modifying DNA accessibility or chromatin structure, mediate long-term effects of gene–environment interactions42,71.
Although retinal asymmetries have not been investigated in chickens yet, left–right differences in the distribution of retinal cells have been reported in other birds, such as starlings and cockatoos119,120. Based on the asymmetrical gene expression in the retina that we have documented, future studies should investigate the role of asymmetrical gene expression in the symmetrical/asymmetrical differentiation of retinal tissues.
To summarise, our data show a molecular association between endogenous and experience-driven lateralisation mediated by light exposure. We identified functional gene networks connected to the vascular system and extracellular matrix as mediators of light-induced asymmetries in the retina, and their connections. The evidence of a temporally defined window of these effects calls for further studies on modulating lateralisation via the molecular pathways that open and close sensitive periods in the developing brain, such as thyroid hormones66,74,121, with important biomedical implications regarding the mechanisms of morphogenesis, ontogenetic plasticity—such as opening/closing of sensitive periods, and for regulating left–right asymmetries.
Supplementary Information
Author contributions
Conceptualisation: S.P., K.A.S., G.V., E.V. Experimental design: S.P., K.A.S., G.V., E.V., P.S., J.L.L. Data collection: P.S., J.L.L., E.V. Brain dissection: J.L.L. Retina dissection, RNA extraction, PCR for sexing: P.S. Data analysis: E.V., P.S., J.G., J.W., P.T., L.J.J., K.A.S. Interpretation of results: E.V., P.S., J.G., J.L.L., P.T., L.J.J., S.P., K.A.S., G.V. Data visualisation: E.V., J.G., J.W. (Fig. 1: E.V., J.W.; Fig. 2: E.V., J.G.; Fig. 3: E.V.; Fig. 4: E.V.). Draft writing: E.V. Funding: K.A.S., S.P., G.V., E.V., J.G. Coordination: E.V., S.P. All authors revised the manuscript.
Funding
This project was supported by BBSRC Grant BB/S003223/1 (EV, JG), Royal Society Grant UF150663 (SP), Novo Nordisk Foundation Grant NNF14CC0001 (LJJ), ERC Horizon 2020 Grant Agreement 833504 SPANUMBRA, PRIN 2017 and ERC-SH4-A (2017PSRHPZ) to GV. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Data availability
All of the sequencing data used are available in the NCBI Short Read Archive under the Bioproject PRJNA743180. All code used in this analysis is available online at https://github.com/peterthorpe5/Gallusggal6_RNAseq.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Elisabetta Versace, Email: e.versace@qmul.ac.uk.
Giorgio Vallortigara, Email: giorgio.vallortigara@unitn.it.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-14963-8.
References
- 1.Versace E, Vallortigara G. Forelimb preferences in human beings and other species: Multiple models for testing hypotheses on lateralization. Front. Psychol. 2015;6:1–9. doi: 10.3389/fpsyg.2015.00233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ströckens F, Güntürkün O, Ocklenburg S. Limb preferences in non-human vertebrates. Laterality. 2013;18:536–575. doi: 10.1080/1357650X.2012.723008. [DOI] [PubMed] [Google Scholar]
- 3.Vallortigara G, Versace E. Laterality at the neural, cognitive, and behavioral levels. In: Call J, editor. APA Handbook of Comparative Psychology: Vol. 1 Basic Concepts, Methods, Neural Substrate, and Behavior. American Psychological Association; 2017. [Google Scholar]
- 4.Rogers LJ, Vallortigara G, Andrew RJ. Divided Brains: The Biology and Behaviour of Brain Asymmetries. Cambridge University Press; 2013. [Google Scholar]
- 5.MacNeilage BPF, Rogers LJ, Vallortigara G. Origins of left and right brain. Sci. Am. 2009;301:60–67. doi: 10.1038/scientificamerican0709-60. [DOI] [PubMed] [Google Scholar]
- 6.Ocklenburg S, Güntürkün O. The Lateralized Brain: The Neuroscience and Evolution of Hemispheric Asymmetries. Elsevier Inc.; 2017. [Google Scholar]
- 7.Vallortigara G, Rogers LJ. Survival with an asymmetrical brain: Advantages and disadvantages of cerebral lateralization. Behav. Brain Sci. 2005;28:575–633. doi: 10.1017/S0140525X05000105. [DOI] [PubMed] [Google Scholar]
- 8.Kurvers RHJM, et al. The evolution of lateralization in group hunting sailfish. Curr. Biol. 2017;27:521–526. doi: 10.1016/j.cub.2016.12.044. [DOI] [PubMed] [Google Scholar]
- 9.Magat M, Brown C. Laterality enhances cognition in Australian parrots. Proc. R. Soc. B Biol. Sci. 2009;276:4155–4162. doi: 10.1098/rspb.2009.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bibost AL, Brown C. Laterality influences cognitive performance in rainbowfish Melanotaenia duboulayi. Anim. Cogn. 2014;17:1045–1051. doi: 10.1007/s10071-014-0734-3. [DOI] [PubMed] [Google Scholar]
- 11.Güntürkün O, Ocklenburg S. Ontogenesis of lateralization. Neuron. 2017;94:249–263. doi: 10.1016/j.neuron.2017.02.045. [DOI] [PubMed] [Google Scholar]
- 12.Vallortigara G, Rogers LJ. A function for the bicameral mind. Cortex. 2020;124:274–285. doi: 10.1016/j.cortex.2019.11.018. [DOI] [PubMed] [Google Scholar]
- 13.Brandler WM, Paracchini S. The genetic relationship between handedness and neurodevelopmental disorders. Trends Mol. Med. 2014;20:84–89. doi: 10.1016/j.molmed.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Trulioff A, Ermakov A, Malashichev Y. Primary cilia as a possible link between left-right asymmetry and neurodevelopmental diseases. Genes. 2017;8:1–24. doi: 10.3390/genes8020048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blum M, Ott T. Animal left–right asymmetry. Curr. Biol. 2018;28:R301–R304. doi: 10.1016/j.cub.2018.02.073. [DOI] [PubMed] [Google Scholar]
- 16.Concha ML, Burdine RD, Russell C, Schier AF, Wilson SW. A nodal signaling pathway regulates the laterality of neuroanatomical asymmetries in the zebrafish forebrain. Neuron. 2000;28:399–409. doi: 10.1016/S0896-6273(00)00120-3. [DOI] [PubMed] [Google Scholar]
- 17.Manns M. It is not just in the genes. Symmetry (Basel) 2021;13:1815. doi: 10.3390/sym13101815. [DOI] [Google Scholar]
- 18.Morandi-Raikova A, Krubeal D, Lorenzi E, Rosa-Salva O, Mayer U. Anatomical asymmetries in the tectofugal pathway of dark-incubated domestic chicks: Rightwards lateralization of parvalbumin neurons in the entopallium. Laterality. 2021;26:163–185. doi: 10.1080/1357650X.2021.1873357. [DOI] [PubMed] [Google Scholar]
- 19.Rogers LJ, Deng C. Light experience and lateralization of the two visual pathways in the chick. Behav. Brain Res. 1999;98:277–287. doi: 10.1016/S0166-4328(98)00094-1. [DOI] [PubMed] [Google Scholar]
- 20.Cuellar-Partida G, et al. Genome-wide association study identifies 48 common genetic variants associated with handedness. Nat. Hum. Behav. 2021;5:59–70. doi: 10.1038/s41562-020-00956-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levin M. Left-right asymmetry in embryonic development: A comprehensive review. Mech. Dev. 2005;122:3–25. doi: 10.1016/j.mod.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 22.Vandenberg LN, Levin M. A unified model for left-right asymmetry? Comparison and synthesis of molecular models of embryonic laterality. Dev. Biol. 2013;379:1–15. doi: 10.1016/j.ydbio.2013.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Basu B, Brueckner M. Cilia: Multifunctional organelles at the center of vertebrate left-right asymmetry. Curr. Top. Dev. Biol. 2008;85:151–174. doi: 10.1016/S0070-2153(08)00806-5. [DOI] [PubMed] [Google Scholar]
- 24.Anvarian Z, Mykytyn K, Mukhopadhyay S, Pedersen LB, Christensen ST. Cellular signalling by primary cilia in development, organ function and disease. Nat. Rev. Nephrol. 2019;15:199–219. doi: 10.1038/s41581-019-0116-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Little RB, Norris DP. Right, left and cilia: How asymmetry is established. Semin. Cell Dev. Biol. 2021;110:11–18. doi: 10.1016/j.semcdb.2020.06.003. [DOI] [PubMed] [Google Scholar]
- 26.Seeger-Nukpezah T, Golemis EA. The extracellular matrix and ciliary signaling. Curr. Opin. Cell Biol. 2012;24:652–661. doi: 10.1016/j.ceb.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goodman L, Zallocchi M. Integrin α8 and Pcdh15 act as a complex to regulate cilia biogenesis in sensory cells. J. Cell Sci. 2017;130:3698–3712. doi: 10.1242/jcs.206201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ning K, et al. Primary cilia in amacrine cells in retinal development. Investig. Ophthalmol. Vis. Sci. 2021;62:1–11. doi: 10.1167/iovs.62.9.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alvarado JA, et al. Developmental distribution of primary cilia in the retinofugal visual pathway. J. Comp. Neurol. 2021;529:1442–1455. doi: 10.1002/cne.25029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tabin CJ, Vogan KJ. A two-cilia model for vertebrate left-right axis specification. Genes Dev. 2003;17:1–6. doi: 10.1101/gad.1053803. [DOI] [PubMed] [Google Scholar]
- 31.McGrath J, Somlo S, Makova S, Tian X, Brueckner M. Two populations of node monocilia initiate left-right asymmetry in the mouse. Cell. 2003;114:61–73. doi: 10.1016/S0092-8674(03)00511-7. [DOI] [PubMed] [Google Scholar]
- 32.McManus IC, Martin N, Stubbings GF, Chung EMK, Mitchison HM. Handedness and situs inversus in primary ciliary dyskinesia. Proc. R. Soc. B Biol. Sci. 2004;271:2579–2582. doi: 10.1098/rspb.2004.2881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Davis EE, Katsanis N. The ciliopathies: A transitional model into systems biology of human genetic disease. Curr. Opin. Genet. Dev. 2012;22:290–303. doi: 10.1016/j.gde.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maizels ET, et al. Cell movements at Hensen’s node establish left/right asymmetric gene expression in the chick. Science. 2009;324:941–945. doi: 10.1126/science.1172478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barth KA, et al. fsi zebrafish show concordant reversal of laterality of viscera, neuroanatomy, and a subset of behavioral responses. Curr. Biol. 2005;15:844–850. doi: 10.1016/j.cub.2005.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schmitz J, Güntürkün O, Ocklenburg S. Building an asymmetrical brain: The molecular perspective. Front. Psychol. 2019;10:982. doi: 10.3389/fpsyg.2019.00982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schmitz J, Metz GAS, Güntürkün O, Ocklenburg S. Beyond the genome—Towards an epigenetic understanding of handedness ontogenesis. Prog. Neurobiol. 2017;159:69–89. doi: 10.1016/j.pneurobio.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 38.Schmitz J, Lor S, Klose R, Güntürkün O, Ocklenburg S. The functional genetics of handedness and language lateralization: Insights from gene ontology, pathway and disease association analyses. Front. Psychol. 2017;8:1–12. doi: 10.3389/fpsyg.2017.01144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rogers LJ. Light input and the reversal of functional lateralization in the chicken brain. Behav. Brain Res. 1990;38:211–221. doi: 10.1016/0166-4328(90)90176-F. [DOI] [PubMed] [Google Scholar]
- 40.Rogers LJ. Light experience and asymmetry of brain function in chickens. Nature. 1982;297:223–225. doi: 10.1038/297223a0. [DOI] [PubMed] [Google Scholar]
- 41.Dadda M, Bisazza A. Prenatal light exposure affects development of behavioural lateralization in a livebearing fish. Behav. Process. 2012;91:115–118. doi: 10.1016/j.beproc.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 42.Chiandetti C, Vallortigara G. Effects of embryonic light stimulation on the ability to discriminate left from right in the domestic chick. Behav. Brain Res. 2009;198:240–246. doi: 10.1016/j.bbr.2008.11.018. [DOI] [PubMed] [Google Scholar]
- 43.Chiandetti C, Regolin L, Rogers LJ, Vallortigara G. Effects of light stimulation of embryos on the use of position-specific and object-specific cues in binocular and monocular domestic chicks (Gallus gallus) Behav. Brain Res. 2005;163:10–17. doi: 10.1016/j.bbr.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 44.Chiandetti C, Lemaire BS, Versace E, Vallortigara G. Early- and late-light embryonic stimulation modulates similarly chicks’ ability to filter out distractors. Symmetry (Basel) 2017;9:84. doi: 10.3390/sym9060084. [DOI] [Google Scholar]
- 45.Chiandetti C, Galliussi J, Andrew RJ, Vallortigara G. Early-light embryonic stimulation suggests a second route, via gene activation, to cerebral lateralization in vertebrates. Sci. Rep. 2013;3:2701. doi: 10.1038/srep02701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Budaev S, Andrew R. Shyness and behavioural asymmetries in larval zebrafish (Brachydanio rerio) developed in light and dark. Behaviour. 2009;146:1037–1052. doi: 10.1163/156853909X404448. [DOI] [Google Scholar]
- 47.Manns M, Güntürkün O. Dual coding of visual asymmetries in the pigeon brain: The interaction of bottom-up and top-down systems. Exp. Brain Res. 2009;199:323–332. doi: 10.1007/s00221-009-1702-z. [DOI] [PubMed] [Google Scholar]
- 48.Kovach JK. Development and mechanisms of behavior in the chick embryo during the last five days of incubation. J. Comp. Physiol. Psychol. 1970;73:392–406. doi: 10.1037/h0030196. [DOI] [PubMed] [Google Scholar]
- 49.Rogers LJ, Bolden SW. Light-dependent development and asymmetry of visual projections. Neurosci. Lett. 1991;121:63–67. doi: 10.1016/0304-3940(91)90650-I. [DOI] [PubMed] [Google Scholar]
- 50.Johnston ANB, Rogers LJ. Light exposure of chick embryo influences lateralized recall of imprinting memory. Behav. Neurosci. 1999;113:1267–1273. doi: 10.1037/0735-7044.113.6.1267. [DOI] [PubMed] [Google Scholar]
- 51.Rogers LJ, Vallortigara G. Lateralized Brain Functions. Humana Press; 2017. [Google Scholar]
- 52.Lorenzi E, Mayer U, Rosa-Salva O, Morandi-Raikova A, Vallortigara G. Spontaneous and light-induced lateralization of immediate early genes expression in domestic chicks. Behav. Brain Res. 2019;368:111905. doi: 10.1016/j.bbr.2019.111905. [DOI] [PubMed] [Google Scholar]
- 53.Mascetti GG, Vallortigara G. Why do birds sleep with one eye open? Light exposure of the chick embryo as a determinant of monocular sleep. Curr. Biol. 2001;11:971–974. doi: 10.1016/S0960-9822(01)00265-2. [DOI] [PubMed] [Google Scholar]
- 54.Skiba M, Diekamp B, Güntürkün O. Embryonic light stimulation induces different asymmetries in visuoperceptual and visuomotor pathways of pigeons. Behav. Brain Res. 2002;134:149–156. doi: 10.1016/S0166-4328(01)00463-6. [DOI] [PubMed] [Google Scholar]
- 55.Chiandetti C. Manipulation of strength of cerebral lateralization via embryonic light stimulation in birds. In: Rogers LJ, Vallortigara G, editors. Lateralized Brain Functions. Springer; 2017. pp. 611–631. [Google Scholar]
- 56.Rogers LJ. Development and function of lateralization in the avian brain. Brain Res. Bull. 2008;76:235–244. doi: 10.1016/j.brainresbull.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 57.Vallortigara G, Cozzutti C, Tommasi L, Rogers LJ. How birds use their eyes: Opposite left-right specialization for the lateral and frontal visual hemifield in the domestic chick. Curr. Biol. 2001;11:29–33. doi: 10.1016/S0960-9822(00)00027-0. [DOI] [PubMed] [Google Scholar]
- 58.Wolpert L. Much more from the chicken’s egg than breakfast—A wonderful model system. Mech. Dev. 2004;121:1015–1017. doi: 10.1016/j.mod.2004.04.021. [DOI] [PubMed] [Google Scholar]
- 59.Vergara MN, Canto-Soler MV. Rediscovering the chick embryo as a model to study retinal development. Neural Dev. 2012;7:1–19. doi: 10.1186/1749-8104-7-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Versace E. Precocial. In: Vonk J, Shackelford T, editors. Encyclopedia of Animal Cognition and Behavior. Springer; 2018. pp. 1–3. [Google Scholar]
- 61.Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. J. Morphol. 1951;88:49–92. doi: 10.1002/jmor.1050880104. [DOI] [PubMed] [Google Scholar]
- 62.Rosa-Salva O, et al. Sensitive periods for social development: Interactions between predisposed and learned mechanisms. Cognition. 2021;213:104552. doi: 10.1016/j.cognition.2020.104552. [DOI] [PubMed] [Google Scholar]
- 63.Dehorter N, Del Pino I. Shifting developmental trajectories during critical periods of brain formation. Front. Cell. Neurosci. 2020;14:1–14. doi: 10.3389/fncel.2020.00283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Versace E, Martinho-Truswell A, Kacelnik A, Vallortigara G. Priors in animal and artificial intelligence: Where does learning begin? Trends Cogn. Sci. 2018;22:963. doi: 10.1016/j.tics.2018.07.005. [DOI] [PubMed] [Google Scholar]
- 65.Aoki N, et al. GABA-A and GABA-B receptors in filial imprinting linked with opening and closing of the sensitive period in domestic chicks (Gallus gallus domesticus) Front. Physiol. 2018;9:1–13. doi: 10.3389/fphys.2018.01837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Batista G, Hensch TK. Critical period regulation by thyroid hormones: Potential mechanisms and sex-specific aspects. Front. Mol. Neurosci. 2019;12:1–9. doi: 10.3389/fnmol.2019.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Takemura Y, et al. Gene expression of Dio2 (thyroid hormone converting enzyme) in telencephalon is linked with predisposed biological motion preference in domestic chicks. Behav. Brain Res. 2018;2:25–30. doi: 10.1016/j.bbr.2018.04.039. [DOI] [PubMed] [Google Scholar]
- 68.McNerney C, Johnston RJ. Thyroid hormone signaling specifies cone photoreceptor subtypes during eye development: Insights from model organisms and human stem cell-derived retinal organoids. Vitamins Hormones. 2021;116:51–90. doi: 10.1016/bs.vh.2021.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Levin M, Johnson RL, Sterna CD, Kuehn M, Tabin C. A molecular pathway determining left-right asymmetry in chick embryogenesis. Cell. 1995;82:803–814. doi: 10.1016/0092-8674(95)90477-8. [DOI] [PubMed] [Google Scholar]
- 70.Zhu L, et al. Cerberus regulates left-right asymmetry of the embryonic head and heart. Curr. Biol. 1999;9:931–938. doi: 10.1016/S0960-9822(99)80419-9. [DOI] [PubMed] [Google Scholar]
- 71.Ververs IAP, de Vries JIP, van Geijn HP, Hopkins B. Prenatal head position from 12–38 weeks. I. Developmental aspects. Early Hum. Dev. 1994;39:83–91. doi: 10.1016/0378-3782(94)90157-0. [DOI] [PubMed] [Google Scholar]
- 72.Cullinan WE, Herman JP, Battaglia DF, Akil H, Watson SJ. Pattern and time course of immediate early gene expression in rat brain following acute stress. Neuroscience. 1995;64:477–505. doi: 10.1016/0306-4522(94)00355-9. [DOI] [PubMed] [Google Scholar]
- 73.Caputto BL, Guido ME. Immediate early gene expression within the visual system: Light and circadian regulation in the retina and the suprachiasmatic nucleus. Neurochem. Res. 2000;25:153–162. doi: 10.1023/A:1007508020173. [DOI] [PubMed] [Google Scholar]
- 74.Lorenzi E, Lemaire BS, Versace E, Matsushima T, Vallortigara G. Resurgence of an inborn attraction for animate objects via thyroid hormone T3. Front. Behav. Neurosci. 2021;15:1–8. doi: 10.3389/fnbeh.2021.675994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rajendra S, Rogers LJ. Asymmetry is present in the thalamofugal visual projections of female chicks. Exp. Brain Res. 1993;92:542–544. doi: 10.1007/BF00229044. [DOI] [PubMed] [Google Scholar]
- 76.Zappia JV, Rogers LJ. Sex differences and reversal of brain asymmetry by testosterone in chickens. Behav. Brain Res. 1987;23:261–267. doi: 10.1016/0166-4328(87)90026-X. [DOI] [PubMed] [Google Scholar]
- 77.Tommasi L, Vallortigara G. Hemispheric processing of landmark and geometric information in male and female domestic chicks (Gallus gallus) Behav. Brain Res. 2004;155:85–96. doi: 10.1016/j.bbr.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 78.Andrew RJ. Behavioural development and lateralization. In: Rogers LJ, Andrew R, editors. Comparative Vertebrate Lateralization. Cambridge University Press; 2002. pp. 157–205. [Google Scholar]
- 79.Khaerunnisa I, Sari E, Ulfah M, Jakaria J, Sumantri C. Avian sex determination based on chromo helicase DNA-binding (CHD) genes using polymerase chain reaction (PCR) Media Peternak. 2013;36:85–90. doi: 10.5398/medpet.2013.36.2.85. [DOI] [Google Scholar]
- 80.Bolger AM, Lohse M, Usadel B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Patro R, Duggal G, Love MI, Irizarry RA, Kingsford C. Salmon provides fast and bias-aware quantification of transcript expression. Nat. Methods. 2017;14:417–419. doi: 10.1038/nmeth.4197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Soneson C, Love MI, Robinson MD. Differential analyses for RNA-seq: Transcript-level estimates improve gene-level inferences. F1000Research. 2015;4:1521. doi: 10.12688/f1000research.7563.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Durinck S, Spellman PT, Birney E, Huber W. Mapping identifiers for the integration of genomic datasets with the R/Bioconductor package biomaRt. Nat. Protoc. 2009;4:1184–1191. doi: 10.1038/nprot.2009.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Manns M, Güntürkün O, Heumann R, Blöchl A. Photic inhibition of TrkB/Ras activity in the pigeon’s tectum during development: Impact on brain asymmetry formation. Eur. J. Neurosci. 2005;22:2180–2186. doi: 10.1111/j.1460-9568.2005.04410.x. [DOI] [PubMed] [Google Scholar]
- 86.Boije H, Ring H, López-Gallardo M, Prada C, Hallböök F. Pax2 is expressed in a subpopulation of Müller cells in the central chick retina. Dev. Dyn. 2010;239:1858–1866. doi: 10.1002/dvdy.22309. [DOI] [PubMed] [Google Scholar]
- 87.Stanke J, Moose HE, El-Hodiri HM, Fischer AJ. Comparative study of Pax2 expression in glial cells in the retina and optic nerve of birds and mammals. J. Comp. Neurol. 2010;518:2316–2333. doi: 10.1002/cne.22335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Raudvere U, et al. G:Profiler: A web server for functional enrichment analysis and conversions of gene lists (2019 update) Nucleic Acids Res. 2019;47:W191–W198. doi: 10.1093/nar/gkz369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Müller AC, et al. Pathway enrichment analysis and visualization of omics data using g:Profiler, GSEA, Cytoscape and EnrichmentMap. Nat. Protoc. 2019;22:924–934. doi: 10.1038/s41596-018-0103-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kahenisa M, Goto S. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 2000;28:27–30. doi: 10.1093/nar/28.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.McGlashan SR, Jensen CG, Poole CA. Localization of extracellular matrix receptors on the chondrocyte primary cilium. J. Histochem. Cytochem. 2006;54:1005–1014. doi: 10.1369/jhc.5A6866.2006. [DOI] [PubMed] [Google Scholar]
- 92.Christensen ST, Morthorst SK, Mogensen JB, Pedersen LB. Primary cilia and coordination of receptor tyrosine kinase (RTK) and transforming growth factor β (TGF-β) signaling. Cold Spring Harb. Perspect. Biol. 2017;9:1–18. doi: 10.1101/cshperspect.a028167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Szklarczyk D, et al. STRING v11: Protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019;47:D607–D613. doi: 10.1093/nar/gky1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Doncheva NT, Morris JH, Gorodkin J, Jensen LJ. Cytoscape StringApp: Network analysis and visualization of proteomics data. J. Proteome Res. 2019;18:623–632. doi: 10.1021/acs.jproteome.8b00702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shannon P, et al. Cytoscape: A software environment for integrated models. Genome Res. 2003;13:2498–2504. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Buschmann JUF, Manns M, Güntürkün O. ‘Let there be light!’ pigeon eggs are regularly exposed to light during breeding. Behav. Process. 2006;73:62–67. doi: 10.1016/j.beproc.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 97.Archer GS, Mench JA. Natural incubation patterns and the effects of exposing eggs to light at various times during incubation on post-hatch fear and stress responses in broiler (meat) chickens. Appl. Anim. Behav. Sci. 2014;152:44–51. doi: 10.1016/j.applanim.2013.12.010. [DOI] [Google Scholar]
- 98.Berardi N, Pizzorusso T, Maffei L. Extracellular matrix and visual cortical plasticity: Freeing the synapse. Neuron. 2004;44:905–908. doi: 10.1016/j.neuron.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 99.Versace E, Ragusa M, Vallortigara G. A transient time window for early predispositions in newborn chicks. Sci. Rep. 2019;9:18767. doi: 10.1038/s41598-019-55255-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Reha RK, et al. Critical period regulation acrossmultiple timescales. Proc. Natl. Acad. Sci. U.S.A. 2020;117:23242–23251. doi: 10.1073/pnas.1820836117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.de Kovel CGF, Lisgo SN, Fisher SE, Francks C. Subtle left-right asymmetry of gene expression profiles in embryonic and foetal human brains. Sci. Rep. 2018;8:12606. doi: 10.1038/s41598-018-29496-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Trimarchi JM, Harpavat S, Billings NA, Cepko CL. Thyroid hormone components are expressed in three sequential waves during development of the chick retina. BMC Dev. Biol. 2008;8:101. doi: 10.1186/1471-213X-8-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Vallortigara G, Regolin L, Marconato F. Visually inexperienced chicks exhibit spontaneous preference for biological motion patterns. PLoS Biol. 2005;3:e208. doi: 10.1371/journal.pbio.0030208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lee HJ, et al. Lateralized feeding behavior is associated with asymmetrical neuroanatomy and lateralized gene expressions in the brain in scale-eating cichlid fish. Genome Biol. Evol. 2017;9:3122–3136. doi: 10.1093/gbe/evx218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Johnson MB, et al. Functional and evolutionary insights into human brain development through global transcriptome analysis. Neuron. 2009;62:494–509. doi: 10.1016/j.neuron.2009.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ramsdell AF. Left-right asymmetry and congenital cardiac defects: Getting to the heart of the matter in vertebrate left-right axis determination. Dev. Biol. 2005;288:1–20. doi: 10.1016/j.ydbio.2005.07.038. [DOI] [PubMed] [Google Scholar]
- 107.Monsoro-Burq AH, Levin M. Avian models and the study of invariant asymmetry: How the chicken and the egg taught us to tell right from left. Int. J. Dev. Biol. 2018;62:63–77. doi: 10.1387/ijdb.180047ml. [DOI] [PubMed] [Google Scholar]
- 108.Wensel TG, Potter VL, Moye A, Zhang Z, Robichaux MA. Structure and dynamics of photoreceptor sensory cilia. Pflugers Arch. Eur. J. Physiol. 2021;473:1517–1537. doi: 10.1007/s00424-021-02564-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gilland E, Miller AL, Karplus E, Baker R, Webb SE. Imaging of multicellular large-scale rhythmic calcium waves during zebrafish gastrulation. Proc. Natl. Acad. Sci. U.S.A. 1999;96:157–161. doi: 10.1073/pnas.96.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Raya Á, et al. Notch activity acts as a sensor for extracellular calcium during vertebrate left-right determination. Nature. 2004;427:121–128. doi: 10.1038/nature02190. [DOI] [PubMed] [Google Scholar]
- 111.Blitzer AL, et al. Primary cilia dynamics instruct tissue patterning and repair of corneal endothelium. Proc. Natl. Acad. Sci. U.S.A. 2011;108:2819–2824. doi: 10.1073/pnas.1016702108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Thanos S, et al. Potential role of Pax-2 in retinal axon navigation through the chick optic nerve stalk and optic chiasm. J. Neurobiol. 2004;59:8–23. doi: 10.1002/neu.20001. [DOI] [PubMed] [Google Scholar]
- 113.Nagashima M, Barthel LK, Raymond PA. A self-renewing division of zebrafish Müller glial cells generates neuronal progenitors that require N-cadherin to regenerate retinal neurons. Development. 2013;140:4510–4521. doi: 10.1242/dev.090738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Salman A, McClements ME, MacLaren R. Insights on the regeneration potential of müller glia on the mammalian retina. Cells. 2021;10:1957. doi: 10.3390/cells10081957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Medland SE, et al. Genetic influences on handedness: Data from 25,732 Australian and Dutch twin families. Neuropsychologia. 2010;47:330–337. doi: 10.1016/j.neuropsychologia.2008.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Paracchini S. Recent advances in handedness genetics. Symmetry (Basel) 2021;13:1792. doi: 10.3390/sym13101792. [DOI] [Google Scholar]
- 117.McManus C. Is any but a tiny fraction of handedness variance likely to be due to the external environment? Laterality. 2021;26:1–5. doi: 10.1080/1357650X.2021.1892126. [DOI] [PubMed] [Google Scholar]
- 118.Mcmanus C. Cerebral polymorphisms for lateralisation: Modelling the genetic and phenotypic architectures of multiple functional modules. Symmetry (Basel) 2022;14:814. doi: 10.3390/sym14040814. [DOI] [Google Scholar]
- 119.Hart NS, Partridge JC, Cuthill IC. Retinal asymmetry in birds. Curr. Biol. 2000;10:115–117. doi: 10.1016/S0960-9822(00)00297-9. [DOI] [PubMed] [Google Scholar]
- 120.Coimbra JP, Collin SP, Hart NS. Topographic specializations in the retinal ganglion cell layer correlate with lateralized visual behavior, ecology, and evolution in cockatoos. J. Comp. Neurol. 2014;522:3363–3385. doi: 10.1002/cne.23637. [DOI] [PubMed] [Google Scholar]
- 121.Hensch TK, Quinlan EM. Critical periods in amblyopia. Vis. Neurosci. 2018 doi: 10.1017/S0952523817000219. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All of the sequencing data used are available in the NCBI Short Read Archive under the Bioproject PRJNA743180. All code used in this analysis is available online at https://github.com/peterthorpe5/Gallusggal6_RNAseq.