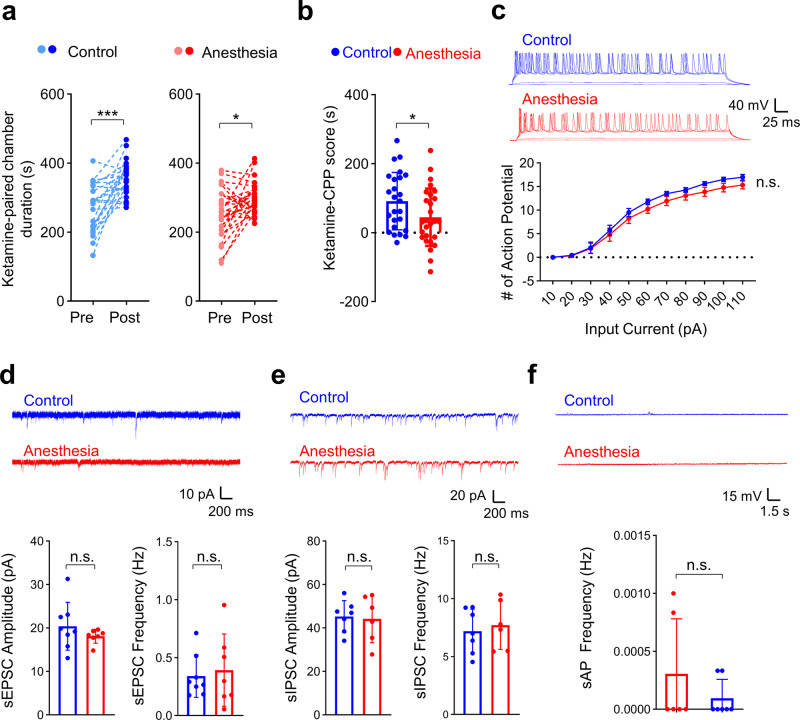
Fig. 5. Female mice are resistant against the increases in addiction behavior and neuronal changes induced by early repeated ketamine anesthesia.
a, b Early repeated ketamine anesthesia reduces low-dose ketamine-induced place preference 1 week after anesthesia injections in female mice (ketamine CPP). a Time spent in the ketamine-paired (white) chamber was significantly increased after conditioning in both groups (control, p < 0.001; paired t test; n = 25; Anesthesia, p = 0.010; paired t test; n = 27). b Summary graph comparing ketamine CPP scores between groups (p = 0.047, Student’s t test). c–f Whole-cell recordings were performed in hippocampal CA1 pyramidal neurons from female mice 1 week after repeated ketamine anesthesia. c Normal intrinsic excitability after early repeated ketamine anesthesia in female mice. The number of action potentials was measured after injecting a series of depolarizing currents under current-clamp conditions (Kruskal–Wallis test and Student’s t test; control, n = 6 mice (total 20 cells); anesthesia n = 6 mice (total 21 cells)). d, e Repeated ketamine anesthesia does not affect spontaneous excitatory synaptic transmission (Welch ANOVA, amplitude: p = 0.190; Student’s t test, frequency: p = 0.698; control, n = 7 mice (total 21 cells); anesthesia, n = 8 mice (total 21 cells)) and spontaneous inhibitory synaptic transmission in female mice (Student’s t test, amplitude: p = 0.833; Student’s t test, frequency: p = 0.643; Control, n = 6 mice (total 22 cells); anesthesia n = 7 mice [total 21 cells]). f Early repeated ketamine anesthesia does not affect spontaneous action potentials (sAPs). sAPs were measured under I = 0 mode (p = 0.600, Kruskal–Wallis test; control, n = 6 mice [total 22 cells]; anesthesia, n = 7 mice [total 20 cells]). Values are presented as means ± SD (*p < 0.05; ***p < 0.001; n.s., not significant).