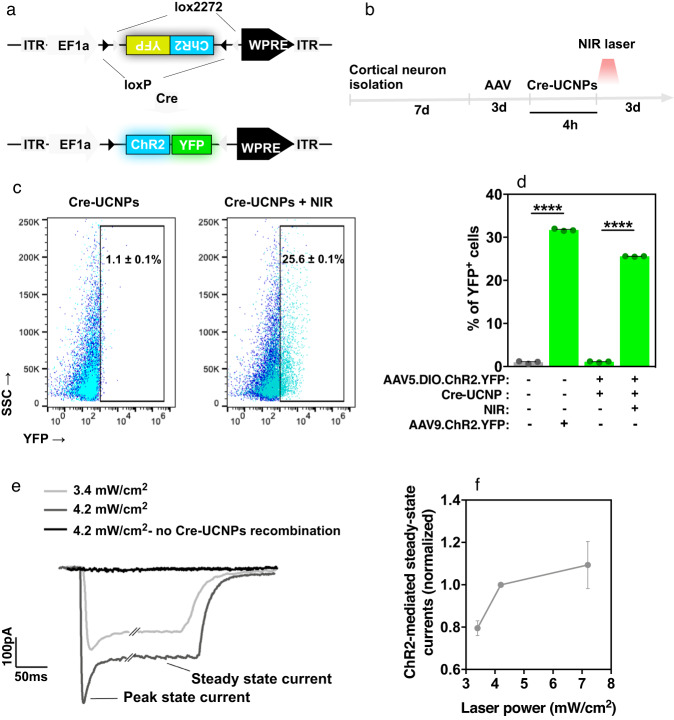
Fig. 5. In vitro gene editing of cortical neurons by Cre-UCNPs followed by cellular activity measurements with optogenetics.
a AAV.dflox.ChR2-YFP cassette used for Cre-mediated expression of ChR2 in cortical neurons. Cre-UCNPs delivery to cells followed by NIR laser activation leads to the inversion of the double-floxed inverted open reading frame and results in the expression of ChR2-YFP fusion protein. b Schematic representation of the experimental setup with cortical neurons. After isolation, cells were cultured for 7 days before treatments. Viral particles (AAV5.dflox.ChR2-YFP) were added to the medium for 3 days. Cells were then exposed to Cre-UCNPs (100 µg/mL) for 4 h, activated by NIR laser (980 nm; 785 mW/cm2; 3 cycles of 5 min irradiation) and allowed to recombine for 3 days. Neurons were activated by a blue light for electrophysiology measurements. c Flow cytometry analyses of ChR2-YFP expression in neuronal cultures. Cells were transfected with Cre-UCNPs and activated or not with a NIR laser. Positive cells were gated based in the basal expression of ChR2-YFP in nontreated cortical neurons (dark blue). d Quantification of recombination by flow cytometry. Results are Mean ± SEM (n = 3). Statistical analyses were performed by one-way ANOVA followed by Bonferroni’s multiple comparisons test: (****), P < 0.0001. e Representative traces of ChR2-mediated currents, showing dependency on the intensity of blue light. Scale bars = 100 pA, 50 ms. Darker trace shows the dependence of Cre-UCNP recombination on the generation of photocurrents. f Voltage-clamp currents generated from different blue-light stimulus. Results are Mean ± SEM (n = 3 independent experiments).