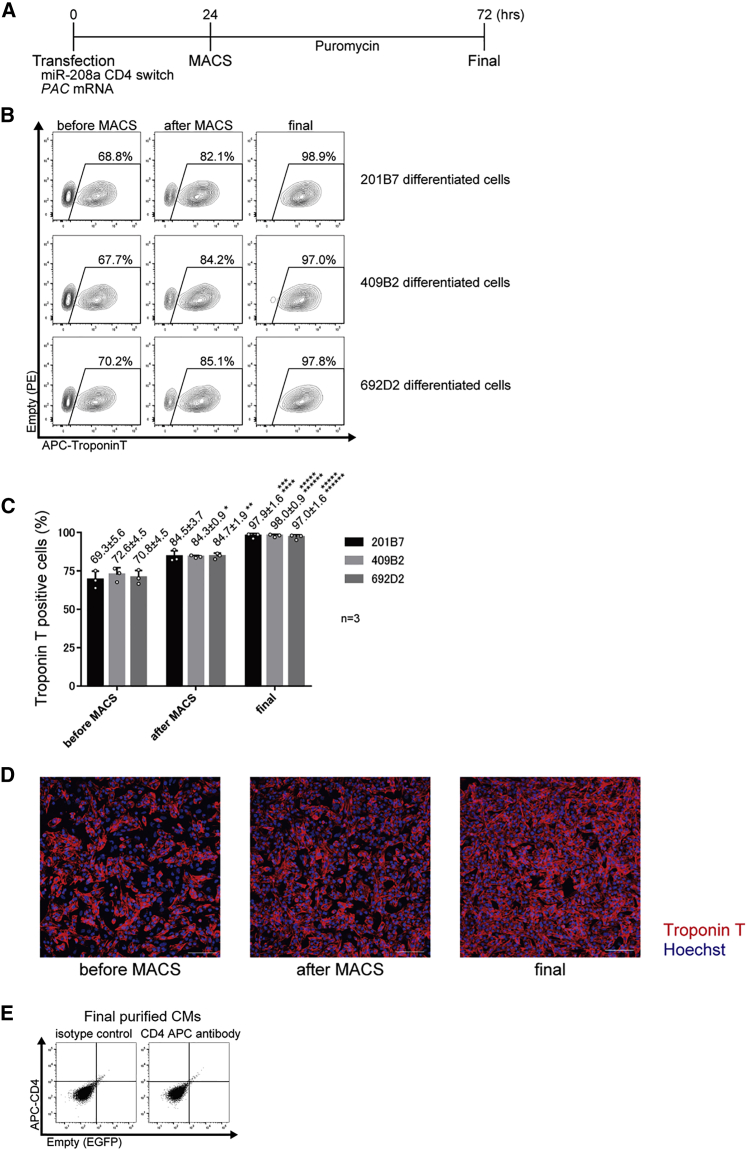
Figure 3.
Purification of iPSC-CMs using miR-208a-CD4 switch and MACS
(A) Schematic procedure for the purification of iPSC-CMs using miR-208a-CD4 switch and MACS. To eliminate untransfected cells, PAC mRNA and puromycin were used.
(B) Representative data of troponin T-positive differentiated 201B7, 409B2, and 692D2 iPSCs before MACS, after MACS, and after purification with puromycin (final). Representative data are shown from three biologically independent experiments. The boxed areas indicate troponin T-positive cells.
(C) The percentage of troponin T-positive differentiated 201B7, 409B2, and 692D2 iPSCs before MACS, after MACS, and after purification. The values are from three biologically independent measurements and denoted as mean ± standard deviation. ∗p < 0.015 versus the corresponding sample before MACS, ∗∗p < 0.01 versus the corresponding sample before MACS, ∗∗∗p < 0.005 versus the corresponding sample after MACS, ∗∗∗∗p < 0.005 versus the corresponding sample before MACS, ∗∗∗∗∗p < 0.001 versus the corresponding sample after MACS, ∗∗∗∗∗∗p < 0.001 versus the corresponding sample before MACS. The data were analyzed by the Bonferroni-Dunn test. Actual values are shown in Table S3.
(D) Fluorescence immunostaining of differentiated 201B7 iPSCs before MACS, after MACS, and after the final purification (n = 3). Representative data from three biologically independent experiments are shown. Red, troponin T; blue, Hoechst. Scale bars, 100 μm.
(E) CD4 expression of the final purified CMs using the miR-208a switch-MACS method. Flow cytometric analysis found no CD4 expression in the final purified CMs after application of the miR-208a-CD4 MACS method and subsequent 48 h administration of puromycin.