Abstract
The conventional chemotherapeutic drugs have many side effects due to their non-selective tissue distribution, reduced drug concentration of the drug at the tumor site, and the drug resistance. To overcome these problems the chemotherapeutic agent should selectively accumulate the tumor site and stays there for a prolonged period of time releasing the payloads in a controlled manner. This can be achieved by the administration of a smart drug delivery system (SDDS) loaded with the active drug molecules. In this work, 5-fluorouracil (5-FU) is loaded into amine functionalised hollow mesoporous silica nanoparticles (HMSN-NH2) and then coated with a biocompatible polydopamine (PDA) to formulate SSDS for 5-FU for pH-sensitive drug release. The physiochemical properties were characterised; the structural morphology was observed by using optical microscope, scanning electron microscope and transmission electron microscope, chemical interaction between the drug and excipients were characterised from Fourier transform infrared spectroscopy, the entrapment efficiency of loaded drug and the pH-dependent drug release rate were evaluated using UV-visible spectroscopy. It was observed that, the drug is compatible with excipients by retaining all the characteristics peaks of 5-FU with negligible changes in the position in all physical mixtures. The PDA coated 5-FU loaded HMSN-NH2 also exhibits a nearly spherical and non-aggregated morphology. The release rate was showed to increase with increase in concentration of structure-directing agent (Triton X 100) in the rate of a maximum release at the end of 72 h in pH 4. The prepared novel PDA coated 5-FU HMSN-NH2 was found to be capable of delivering the anti-cancer drug 5-FU specifically at the tumor site in a pH-dependent stimuli-responsive manner. It also showed a controlled release for a period of 72 h. The enhanced cytotoxicity against HeLa cell line were found for the formulated SSD form.
Keywords: Smart drug delivery system, Optical microscope, Hollow mesoporous silica nanoparticles, Polydopamine, 5-Fluorouracil, Scanning electron microscope and transmission electron microscope, FTIR spectroscopy, UV-visible spectroscopy
Graphical abstract

Smart Drug Delivery System; Optical microscope; Hollow mesoporous silica nanoparticles; Polydopamine; 5-Fluorouracil; Scanning Electron microscope and transmission electron microscope; FTIR spectroscopy; UV-visible spectroscopy.
1. Introduction
Cancer is one of the largest causes of morbidity and mortality worldwide [1]. Global Cancer Statistics 2020 reports shows that an estimation of 19.3 million new cancer cases (18.1 million excluding nonmelanoma skin cancer) and almost 10.0 million cancer deaths (9.9 million excluding nonmelanoma skin cancer) occurred worldwide, in 2020 [1]. Female breast cancer has exceeded lung cancer as the most commonly diagnosed cancer, with an estimated 2.3 million new cases (11.7%), followed by lung (11.4%), colorectal (10.0 %), prostate (7.3%), and stomach (5.6%) cancers. Lung cancer remained the leading cause of cancer death, with an estimated 1.8 million deaths (18%), followed by colorectal (9.4%), liver (8.3%), stomach (7.7%), and female breast (6.9%) cancers. These cancers can be treated, if detected at an early stage. The treatment of cancer usually confined to chemotherapy and radiation and surgery, which usually involving a lot of side effects [2]. The conventional treatment options are less efficient and causing side effects because there is a non-selective tissue distribution of the drug, reduced drug concentration of the drug at the tumor site, and the drug resistance caused by the repeated exposure of the tumor to the chemotherapeutic agents. To overcome these problems the chemotherapeutic agent should selectively accumulate the tumor site and stays there for a prolonged period of time releasing the payloads in a controlled manner [2]. This can be achieved by the administration of a smart drug delivery system (SDDS) loaded with the active drug molecules.
SDDS ensures a controlled release of drugs in the recognized vicinity of the target site sparing the non-targets in response to a stimulus. SDSS method can also reduce the dosage administration frequency and achieve the finest patient compliance, since it release its payloads in a smart way. The physicochemical properties of the delivery systems are customized to develop a stimuli-responsive system that can deliver the therapeutic molecule on demand [3]. So by incorporating the active drug into a SDDS we can ensure that the drug will not extravasate before it senses the stimuli. At the same time loading drug into a hollow mesoporous silica nanoparticles (HMSN) possess some superior features than its pristine nature. In HMSN a large hollow cavity inside will help to increase the amount of drug-loaded into it which is more advantageous. Surface modification of drug-loaded HMSN with polymers, ligands, supramolecules and quantum dots can be done to make it a stimuli-responsive active targeting drug delivery system (5). Here, A nature-inspired biopolymer polydopamine (PDA) that has an excellent biocompatibility and bio adhesion with free radical scavenging activity has been explored as a coating material with stimuli-responsive properties [4]. PDA is a mimic of the specialized adhesive foot protein Mefp-5 (Mytilus edulis foot protein) secreted by mussels which is having an excellent bio adhesion and biocompatibility is used to modify the surface of the HMSN [5]. Here PDA act as gatekeepers on the mesopores to prevent the premature release of the drug.
5 Fluorouracil (5-FU), is an antineoplastic agent which is used alone or in combination with the chemotherapy regimens for the treatment of a wide range of different cancers [6]. The conventional treatment options of 5-FU are less efficient and cause side effects because there is the non-selective tissue distribution of the drug, reduced drug concentration of the drug at the tumour site, and the drug resistance caused by the repeated exposure of the tumour to the chemotherapeutic agents [6]. The amine functionalization of HMSNs for enhanced drug loading of 5-FU was already reported by Xiaodong et al. [6]. In this work, 5-FU is loaded into amine-functionalized HMSN and then coated with a biocompatible PDA to formulate a smart drug delivery system for 5-FU for pH-sensitive drug release. The physicochemical properties were characterized; the structural morphology was observed by using an optical microscope, scanning electron microscope and transmission electron microscope, Chemical interaction between the drug and excipients was characterized by FTIR spectroscopy, the entrapment efficiency of loaded drug and the pH-dependent drug release rate were evaluated using UV-visible spectroscopy. Further, the Functional validation of the formulated drug was evaluated by long-term cytotoxicity against HeLa cancer cell lines.
2. Experimental methods
2.1. Materials
5-Fluorouracil (5-FU) a white crystalline powder with a molecular weight of 130.08 g/mol, was purchased from Yarrow Chem Products, Mumbai with 99.9% purity. Other chemicals (tetraethyl orthosilicate, Triton X 100, dopamine hydrochloride, eudragit s 100, 3 aminopropyl triethoxysilane, tris buffer) used in the study were in the analytical grade were purchased from TCI Chemicals, India.
2.2. Development of polydopamine coated 5-fluorouracil loaded hollow mesoporous silica nanoparticles
Hollow Mesoporous Silica Nanoparticles (HMSN) were developed by using Eudragit S 100 nanoparticles as hardcore and Triton X 100 as a structure-directing agent [7]. The synthesized HMSN was functionalized with an amine to increase its loading capacity [6]. For that, 25 μl of (3-aminopropyl) triethoxysilane (APTES) was added to a dispersion of 150 mg of HMSNs and ethanol (20 mL) and stirred for 24 h at 650 rpm. The amine-functionalized HMSN-NH2 were collected by cold centrifugation followed by ethanol and deionized water washing and drying in a vacuum desiccator. The loading of 5-FU was carried out by ultrasonically soaking HMSN-NH2 in a solution of drug in deionized water and the dispersion was stirred at room temperature for 24 h under magnetic stirring at 650 rpm. The 5-FU loaded HMSN-NH2 were separated by cold centrifugation at 13000 rpm and washed with deionized water. It was then dried in a vacuum desiccator [[6], [8], [9]].
Further ultrasonic dispersion method was used to coat solid Polydopamine (PDA) on 5-FU loaded HMSN-NH2. 5-FU loaded HMSN-NH2 ultrasonically dispersed in 50 ml of Tris-buffer (pH 8.5, 10 mmol/L) followed by stirring in a magnetic stirrer at 650 rpm, and then dopamine hydrochloride (25 mg) was added. The mixture was stirred for 24 h in the dark. The solid of PDA coated 5-FU loaded HMSN-NH2 was centrifugated (13,000 rpm, 10 min), and washed with water to remove the unpolymerized dopamine hydrochloride. PDA-coated 5-FU loaded HMSN-NH2 was stored in 4 °C before releasing experiments [10].
2.3. Entrapment efficiency
The drug entrapment efficiency was determined in all the formulations prepared with and without amine functionalization of HMSNs (with formulation code amine functionalization (F1a, F2a, F3a, F4a) and non-amine functionalized HMSNs (F1b, F2b, F3b, F4b) having 0.5, 1.0, 1.5, 2.0 mg Triton X 100 concentration respectively) by UV spectroscopic estimation (266 nm) of the filtered solution (0.22 μm syringe filter) after centrifuging the obtained supernatant of formulations at different concentrations. The entrapment efficiency (EE) was calculated as follows [11]:
2.4. FT-IR spectroscopy
FTIR spectroscopy is a qualitative analytical technique, which offers the possibility of detecting chemical interactions between drug and excipient in the formulation. The vibrational spectra of neat and binary AMB were recorded by SHIMADZU’s IRAffinity’s 1 spectrometer at room temperature. The measurements were carried out in the wavenumber range from 400 to 4000 cm−1 [12,13,14].
2.5. Morphological analysis
2.5.1. Optical microscopy
The surface morphology of the prepared drug-loaded HMSNs and PDA coated 5-FU loaded HMSN-NH2 were observed under Labomed optical microscope with 45X magnification.
2.5.2. Scanning electron microscopy
The surface morphology of the prepared drug-loaded HMSNs and PDA coated 5-FU loaded HMSN-NH2 were examined by scanning electron microscope - JEOL Model JSM – 6390LV (JEOL Ltd, Tokyo, Japan). The samples under study were placed on the aluminum holder using carbon tape. Then the samples were electrically grounded with either gold or silver. After that, the samples were dried in an oven at 600 C for 3 h. Then samples were loaded onto the SEM holder to record the SEM data [7,10,12,15,16].
2.5.3. Transmission electron microscopy
The morphologies of PDA coated 5-FU loaded HMSN-NH2 were investigated by transmission electron microscopy (TEM) on an H-7500 transmission electron microscope (Hitachi, Japan) operated at 80 kV. Before TEM measurement, the sample for TEM microscopy was prepared by dropping a dispersed solution on a carbon film supported by a copper grid (230 meshes) and dried by infrared light. Then the sample was loaded for TEM measurement [7].
2.6. Particle size analysis
The particle size distribution of drug-loaded HMSNs and PDA coated 5-FU loaded HMSN-NH2 dispersions were determined by Dynamic light scattering using a Zeta sizer. To measure the zeta potential, a small quantity of sample was injected into a cell containing two electrodes that were used to create an induced electric field. Once the electric field was applied, the particles move towards either the anode or cathode depending on whether the surfaces are positively or negatively charged. The direction of the motion indicates a positive or negative charge [[17], [18], [19]].
2.7. MTT assay
In vitro cytotoxicity study of the formulation was carried out by MTT assay using HeLa cells. HeLa cells (Cervical cancer cell line) were initially procured from National Centre for Cell Sciences (NCCS), Pune, India, and maintained in Dulbecos modified Eagles medium (Himedia). The cell line was cultured in a 25 cm2 tissue culture flask with DMEM supplemented with 10% FBS, L-glutamine, sodium bicarbonate, and an antibiotic solution containing: penicillin (100 U/ml), streptomycin (100 μg/ml), and amphotericin B (2.5 μg/ml). Cultured cell lines were kept at 37 °C in a humidified 5% CO2 incubator (Galaxy® 170 Eppendorf, Germany). The viability of cells was evaluated by direct observation of cells by an inverted phase-contrast microscope and followed by the MTT assay method [[4], [20], [21], [19], [22], [23], [24], [25]].
3. Result and discussion
To achieve a better therapeutic efficacy with the least side effects in the treatment of cancer, the anti-cancer agent 5-Fluorouracil has been loaded into HMSN. Different concentrations of the structure-directing agent, i.e., Triton X 100 (0.5 g, 1 g, 1.5 g, and 2 g) were used. The formulation of 5- fluorouracil loaded HMSNs were carried out in 5 stages; synthesis of eudragit S 100 nanoparticles, formulation of eudragit S 100 nanoparticles coated with silica, chemical etching: formulation of HMSN by removing Triton X 100 template and eudragit S 100 core, amine functionalization on the surface of the HMSN and preparation of 5-FU loaded HMSN. Henceforth two sets of 5-FU loaded HMSN were prepared with and without amine functionalization.
The polydopamine (PDA) coating was carried out for getting a pH-responsive smart drug delivery system. The mussel-inspired polydopamine was used for coating as it resembles the mussel foot proteins Mefp-5 (Mytilus edulis foot protein). PDA helps for bioadhesive surface coating resembling mussels due to the presence of catechol and amine groups. The 5 -FU loaded HMSNs were dispersed in tris HCl buffer under stirring followed by the addition of dopamine HCl. The procedure was carried out under darkness as PDA is light-sensitive and a schematic diagram of the formulation of PDA coated 5-FU loaded HMSN was depicted in Figure 1. The unpolymerized dopamine HCl was separated and the PDA coated 5 FU loaded HMSNs were collected, dried, and stored at 4 °C
Figure 1.
Schematic diagram showing the loading of 5-FU in HMSN and subsequent coating with PDA polymer to formulate pH-sensitive SDD.
3.1. Drug –excipient compatibility studies
The drug-polymer compatibility study was performed by FTIR spectroscopy. The spectrum obtained from the physical mixture of 5-FU with excipients including tetraethyl orthosilicate (TEO), Triton X 100, 3- aminopropyl triethoxysilane (APTES), dopamine HCl and PDA coated 5-FU loaded HMSN was compared with that of pure drug and shown in Figure 2.
Figure 2.
FTIR spectra of a)5-FU, b) PDA coated 5-FU loaded HMSN c) Excipients; tetraethyl orthosilicate (TEO), Triton X 100, 3- aminopropyl triethoxysilane (APTES) and dopamine HCl.
5- fluorouracil was characterized by 6 significant peaks due to N–H stretching at 3173 cm−1, C=O stretching at 1720 cm−1, C–N stretching at 1649 cm−1, and C–H stretching in a plane at 1243 cm−1, C–O stretching at 951 cm−1 and C–F at 838 cm−1. All the major peaks of 5-FU were observed in the physical mixture of drug and polymers with negligible changes in the position. All the assignments to FTIR spectra of samples under study were listed in Table 1. This suggests that there was no interaction between drug and polymer.
Table 1.
Assignments to FTIR Spectra of a) 5-FU, b) PDA coated 5-FU loaded HMSN c) Excipients; tetraethyl orthosilicate (TEO), Triton X 100, 3- aminopropyl triethoxysilane (APTES) and dopamine HCl.
| Wavenumber (cm−1) |
Assignments | |||||
|---|---|---|---|---|---|---|
| 5-FU | Excipients |
PDA coated 5-FU loaded HMSN |
||||
| TEO | Triton 100 | APTES | DHCl | |||
| 3469 | 3447 | O–H Bending | ||||
| 3173 | 3335 | 3335 | 3173 | N–H (Stretching) free | ||
| 2977 | 2961 | 3055 | C–H Bending | |||
| 2938 | O–H Stretching | |||||
| 2889 | 2873 | C–H Stretching | ||||
| 2038 | ||||||
| 1720 | 1757 | 1627 | C=O (Stretch) | |||
| 1649 | 1506 | C–N (Stretch) | ||||
| 1635 | 1635 | C–H Stretching | ||||
| 1615 | 1615 | N–H Bending | ||||
| 1613 | 1605 | C=C Stretching | ||||
| 1503 | ||||||
| 1464 | 1448 | C–H Bending (methylene) | ||||
| 1398 | 1348 | 1331 | ||||
| 1243 | 1287 | 1243 | C–H (in plane) | |||
| 951 | 1266 | 1259 | 1088 | 1088 | 1100 | C–O Stretching |
| 1111 | 917 | |||||
| 834 | 862 | |||||
| 838 | 838 | C–F | ||||
| 814 | ||||||
| 785 | C–H Bending | |||||
3.2. Surface morphology
The morphological examinations of 5-FU loaded HMSN-NH2 and PDA coated 5-FU loaded HMSN-NH2 were carried out using optical microscopy and scanning electron microscopy. The optical microscopic images were collected Labomed optical microscope and were shown in Figure 3a & b. The microscopic image of 5-FU loaded HMSN-NH2 (Figure 3a) revealed that the particles of drug-loaded HMSN were nearly spherical and non-aggregated. The surface topography of the particles was found to be highly porous, this may be due to amine functionalization. While the PDA coated 5-FU loaded HMSN-NH2 also exhibits a nearly spherical and non-aggregated (Figure 3b). The change in the colour of the particle from white to amber clearly indicated the presence of PDA coating on the surface of drug-loaded HMSNs.
Figure 3.
Optical microscopic image of a) 5-FU loaded HMSN-NH2 b) PDA coated 5-FU loaded HMSN-NH2 at 40x magnification.
The electron micrographs showed the spherical, porous and homogenous nature of the particle of 5-FU loaded HMSN-NH2 as shown in Figure 4. a. The porous nature enhanced the drug loading capacity of HMSNs. At the same time, the electron micrographs of PDA coated 5-FU loaded HMSN-NH2 clearly visualize the deposition of polydopamine over the highly porous HMSNs as shown in Figure 4. b. The deposition of PDA may greatly influence the pH-dependent release of 5-FU.
Figure 4.
SEM image of 5-FU loaded HMSN-NH2 and PDA coated 5-FU loaded HMSN-NH2 at different magnification.
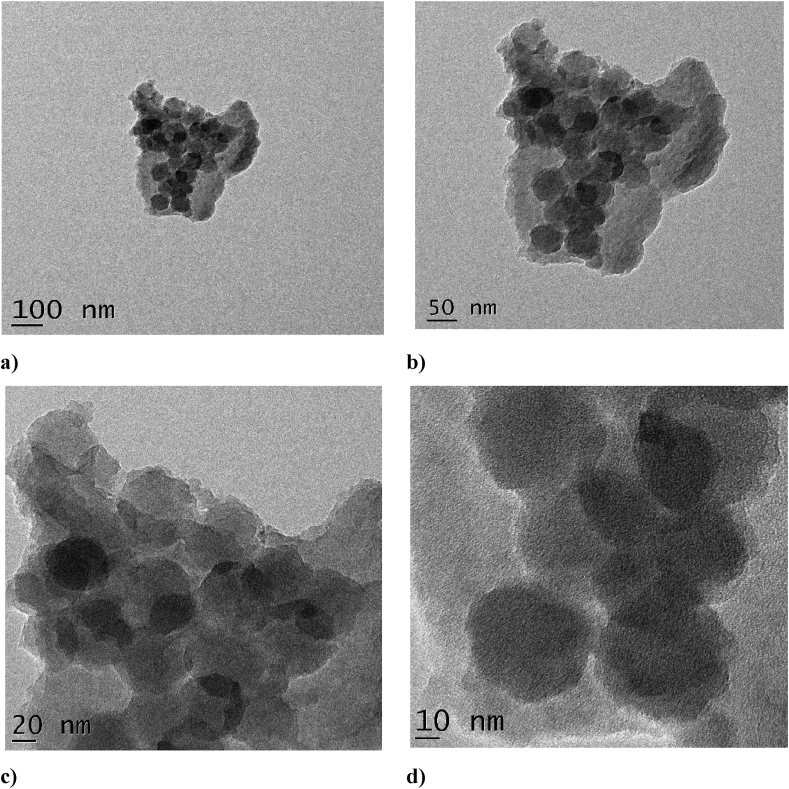
Further, the TEM analysis was carried out to ensure the higher porous and spherical nature of PDA-coated 5-FU loaded HMSN-NH2. As can be seen from Figure 5 the rough surface around the nanoparticles indicates the deposition of polydopamine over HMSNs. This clearly indicates the successful homogeneous coating of PDA over the 5-FU-loaded HMSN. The image obtained from TEM depicts that the coated nanoparticles have a size range of roughly 150–250 nm.
Figure 5.
TEM image of PDA coated 5-FU loaded HMSN-NH2 at a) 100 nm b) 50 nm c) 20 nm and d) 10 nm magnifications.
3.3. Particle size analysis
The particle size of the 5-FU loaded HMSN-NH2 and PDA coated 5-FU loaded HMSN-NH2 were analyzed by Zeta sizer. The particle size of the amine-functionalized drug-loaded HMSNs was found to be 119.5 nm with a polydispersity index of 0.196 (Figure 6a), while the particle size of PDA coated 5-FU loaded HMSN-NH2 was found to be increased to 145 nm with a polydispersity index 0.549 (Figure 6b).
Figure 6.
Particle size and Particle size distribution of a) 5-FU loaded HMSN-NH2 b) PDA coated 5-FU loaded HMSN-NH2.
The measured zeta potential 5-FU loaded HMSN-NH2 and PDA coated 5-FU loaded HMSN-NH2 were shown in Figure 7 a & b respectively. It was found that the zeta potential of PDA coated 5-FU loaded HMSN-NH2 was −29.6 mV (Figure 7b). The zeta potential of 5-FU loaded HMSN-NH2 without PDA coating was −21.1 mV (Figure 7a). The high values after PDA coating confirm that the nanoparticulate dispersion of HMSNs was more stable after the coating with PDA.
Figure 7.
The measured Zeta potential of a) 5-FU loaded HMSN-NH2 b) PDA coated 5-FU loaded HMSN-NH2.
3.4. Entrapment efficiency
The entrapment efficiency of the formulations prepared with and without amine functionalization was determined and shown in Figure 8. The entrapment efficiency was found to be significantly higher for formulations prepared with amine functionalization (F1a, F2a, F3a, F4a) when compared with non-amine functionalized HMSNs (F1b, F2b, F3b, F4b). The enhancement of entrapment efficiency might be due to the hydrophilic nature of both the drug and amine-functionalized surface of HMSNs with opposite charges. It was also observed that the entrapment efficiency increases with an increase in the concentration of the structure-directing agent (Triton X 100). F4a containing 2 mg of Triton X 100 exhibited the highest entrapment efficiency of 35.23 ± 0.75.
Figure 8.
Entrapment Efficiency of different formulations.
3.5. pH-sensitive drug release releasing
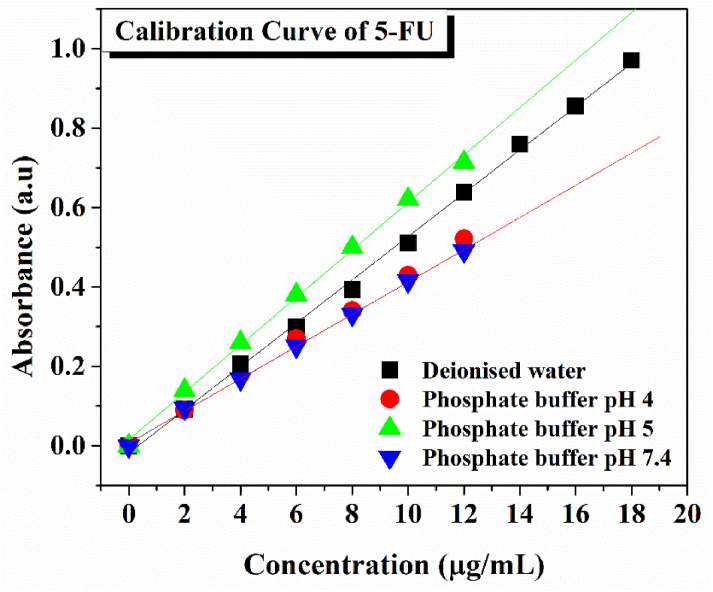
The calibration curve of 5-FU was plotted at different concentrations of drug in deionized water, Phosphate buffer pH 4,5, and 7.4 was measured at 266 nm and shown in Figure 9. All the curves obeyed Beer-Lambert’s law with a linear relationship with an R2 value of 0.9995.
Figure 9.
Calibration curve of 5-FU in deionized and phosphate buffer with different pH.
The Polydopamine coated 5-FU loaded HMSNs; PdF1a, PdF2a, PdF3a, and PdF4a having 0.5, 1.0, 1.5, 2.0 mg Triton X 100 concentrations were subjected to in vitro drug release using dialysis bag in Phosphate buffer pH 4, 5 and 7.4 and the pH-dependent drug release curve were depicted in Figure 10. This study was performed to assure the pH-dependent release of 5-FU from PDA-coated HMSNs. All the formulations showed a pH-dependent stimuli response pattern in the order of pH 4 > pH 5 > pH 7.4. The release rate was showed to increase with increase in concentration of structure-directing agent (Triton X 100) in the rate of a maximum release PdF1a - 52.2%, PdF2a - 62.0%, PdF3a - 69.1% and PdF4a - 80.5% at the end of 72 h in pH 4. Hence, we can use this formulation as a smart drug delivery system to deliver the drug exactly at the tumor site having low pH in a controlled release manner.
Figure 10.
In vitro drug release from PDA coated 5-FU loaded HMSNs.
3.6. Biological evaluation – cytotoxicity
In vitro analysis of cervical cancer cells was done using an MTT assay for examining the cytotoxic effect of 5-FU, 5-FU loaded HMSNs and PDA coated 5-FU loaded HMSNs (PdF4a). MTT assay has been done in different concentrations (6.25, 12.5, 25, 50, 100 μg/mL) of all samples. The HeLa cells treated with drugs showed morphological changes and were found to be detached from the surface as shown in Figure 11. Figure 12 shows the relationship between % of cytotoxicity and the concentration of drugs in μg/mL. The 5-FU molecule displays anticancer activity with the IC50 value of 40.7 μg/mL, while the anticancer activity is found to increase in 5-FU loaded HMSNs (IC50 = 31.3 μg/mL) and enhanced in PDA coated 5-FU loaded HMSNs (PdF4a) with the IC50 value of 20.29 μg/mL. The above results show that drug loading in HMSN and PDA coating thereafter aids cellular uptake of the 5-FU drug of interest and allows targeted drug delivery [19, 21].
Figure 11.
Microscopic observations of In vitro cytotoxicity with free 5-FU, 5-FU loaded HMSN and PDA coated 5-FU loaded HMS solution.
Figure 12.
Graphical representation of the cytotoxic effect of free 5-FU, 5-FU loaded HMSN and PDA coated 5-FU loaded HMS solution.
4. Conclusion
In this paper, an antineoplastic agent 5-FU was loaded into amine-functionalized HMSN and then coated with a biocompatible PDA to formulate a smart drug delivery system for pH-sensitive drug release. The chemical compatibility of 5-fu with excipients and the structural morphology of the formulated SDD was investigated by means of various experimental techniques such as FTIR spectroscopy, optical microscope, scanning electron microscope, and transmission electron microscope. Further, the entrapment efficiency of loaded drugs and the pH-dependent drug release rate were evaluated using UV-visible spectroscopy. All the performed studies indicated the drug is compatible with excipients by retaining all the characteristic peaks of 5-FU with negligible changes in the position in all physical mixtures. The PDA-coated 5-FU loaded HMSN-NH2 also exhibits a nearly spherical and non-aggregated morphology. The novel Polydopamine coated 5-Fluorouracil HMSN was found to be capable of delivering the anti-cancer drug 5-FU specifically at the tumor site in a pH-dependent stimuli-responsive manner. It also showed a controlled release for a period of 72 h. Hence this novel SDD System can be a good replacement for conventional chemotherapy. By this approach, we can reduce the unwanted side effects caused by the anti-cancer agents along with an increased therapeutic efficacy. The future perspectives include pharmacokinetic studies and clinical trials for developing a clinically viable formulation.
Declarations
Author contribution statement
Shamla Cheralayikkal: Performed the experiments.
Manoj K: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data.
Safna Hussan K.P: Analyzed and interpreted the data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data will be made available on request.
Declaration of interest’s statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
We gratefully acknowledge the staff at the College of pharmaceutical sciences, Govt medical college, Kozhikode, for their constant encouragement and support, which has provided a good and smooth basis for this research.
Contributor Information
K. Manoj, Email: manoj4880@gmail.com.
K.P. Safna Hussan, Email: safnahussain2@gmail.com.
References
- 1.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries, CA. Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Chidambaram M K.K., Manavalan R. Nanotherapeutics to overcome conventional cancer chemotherapy limitations. J. Pharm. Pharmaceut. Sci. 2011;14:66–77. doi: 10.18433/j30c7d. [DOI] [PubMed] [Google Scholar]
- 3.Pérez-Herrero E., Fernández-Medarde A. Advanced targeted therapies in cancer: drug nanocarriers, the future of chemotherapy. Eur. J. Pharm. Biopharm. 2015;93:52–79. doi: 10.1016/j.ejpb.2015.03.018. [DOI] [PubMed] [Google Scholar]
- 4.Elayaperumal M., Venugopal K. Green synthesis of silver nanoparticles using piper nigrum concoction and its anticancer activity against MCF-7 and Hep-2 cell lines. J. Antimicrob. Agents. 2016;2:8–12. [Google Scholar]
- 5.Piggott A.M., Karuso P. 2005. Marine-derived Indole Alkaloids and Their Biological and Pharmacological Activities. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.She X., Chen L., Li C., He C., He L., Kong L. Functionalization of hollow mesoporous silica nanoparticles for improved 5-fu loading. J. Nanomater. 2015;2015 [Google Scholar]
- 7.Huang X., Young N.P., Townley H.E. Characterization and comparison of mesoporous silica particles for optimized drug delivery. Nanomater. Nanotechnol. 2014;4:1–15. [Google Scholar]
- 8.Ch V., Sailaja K. Preparation of ibuprofen-loaded eudragit S100 nanoparticles by solvent evaporation technique. Int. J. Pharma Sci. Res. 2014;5:376–384. https://www.ijpsr.info/docs/IJPSR14-05-07-003.pdf [Google Scholar]
- 9.Zheng Q., Lin T., Wu H., Guo L., Ye P., Hao Y., Guo Q., Jiang J., Fu F., Chen G. Mussel-inspired polydopamine coated mesoporous silica nanoparticles as pH-sensitive nanocarriers for controlled release. Int. J. Pharm. 2014;463:22–26. doi: 10.1016/j.ijpharm.2013.12.045. [DOI] [PubMed] [Google Scholar]
- 10.Safna Hussan K.P., Mohamed Shahin T., Deshpande S.K., V Jinitha T., Kolte J. Development of ion conducting ionic liquid-based gel polymer electrolyte membrane PMMA/BMPyr . TFSI – with improved electrical , optical , thermal and structural properties. Solid State Ionics. 2017;310:166–175. [Google Scholar]
- 11.Gao Y., Chen Y., Ji X., He X., Yin Q., Zhang Z., Shi J., Li Y. Controlled intracellular release of doxorubicin in multidrug-resistant cancer cells by tuning the shell-pore sizes of mesoporous silica nanoparticles. ACS Nano. 2011;5:9788–9798. doi: 10.1021/nn2033105. [DOI] [PubMed] [Google Scholar]
- 12.Jinitha T.V., Safna Hussan K.P., Mohamed Shahin T., Purushothaman E. The interplay between the fragility and mechanical properties of styrene–butadiene rubber composites with unmodified and modified sago seed shell powder. J. Appl. Polym. Sci. 2020:1–13. [Google Scholar]
- 13.Safna Hussan K.P., Hisana Asharaf T.P., Parammal Hamza Shahina, Thirumangalath Parameswaran Anjali, Shahin Thayyil Mohamed, Karuvanthol Muraleedharan. Density Funct. Theory Calc. Fig. 2019. DFT and molecular docking studies of a set of non-steroidal anti-inflammatory drugs: propionic acid derivatives; pp. 1–10. [Google Scholar]
- 14.Safna Hussan K.P., Moidu Haroon Hussain, Shahin Thayyil Mohamed, Jinitha T.V., Antony Anu, Govindaraj G. Physisorption mechanism in a novel ionogel membrane based CO2 gas sensor. J. Mater. Sci. Mater. Electron. 2021;32:25164–25174. [Google Scholar]
- 15.Sangeetha K.G., Aravindakshan K.K., Hussan K.P.S. Insight into the theoretical and experimental studies of 1-phenyl-3- phenylthiosemicarbazone – a potential NLO material. J. Mol. Struct. 2017;1150:135–145. [Google Scholar]
- 16.Safna Hussan K.P., Mohamed Shahin T., V Jinitha T., Jayant K. Development of an ionogel membrane PVA/[EMIM] [SCN] with enhanced thermal stability and ionic conductivity for electrochemical application. J. Mol. Liq. 2019;274:402–413. [Google Scholar]
- 17.Doub W.H., Adams W.P., Spencer J.A., Buhse L.F., Nelson M.P., Treado P.J. Raman chemical imaging for ingredient-specific particle size characterization of aqueous suspension nasal spray formulations: a progress report. Pharm. Res. 2007;24:934–945. doi: 10.1007/s11095-006-9211-2. [DOI] [PubMed] [Google Scholar]
- 18.Dhoranwala K.A., Shah P., Shah S. Formulation optimization of rosuvastatin calcium-loaded solid lipid nanoparticles by 32 full-factorial design. NanoWorld J. 2016;1:112–121. [Google Scholar]
- 19.Christopher Jeyaseelan S., Milton Franklin Benial A. Spectroscopic characterization, DFT studies, molecular docking and cytotoxic evaluation of 4-nitro-indole-3-carboxaldehyde: a potent lung cancer agent. J. Mol. Recogn. 2021;34 doi: 10.1002/jmr.2872. [DOI] [PubMed] [Google Scholar]
- 20.Mohammed A.M.E. Estimation of the active components in gum Arabic collected from Western Sudan. Int. J. Sci. Res. 2017;6:1262–1282. [Google Scholar]
- 21.Shyni V., Leenaraj D.R., Ittyachan R., Joseph L., Sajan D. Spectroscopic, density functional theoretical study, molecular docking, and in vitro studies based on anticancer activity studies against A549 lung cancer cell line of diphenylhydantoin adsorbed on AuNPs surface. J. Mol. Recogn. 2021;34:1–19. doi: 10.1002/jmr.2916. [DOI] [PubMed] [Google Scholar]
- 22.More G.K., Makola R.T. In-vitro analysis of free radical scavenging activities and suppression of LPS-induced ROS production in macrophage cells by Solanum sisymbriifolium extracts. Sci. Rep. 2020;10:1–9. doi: 10.1038/s41598-020-63491-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Owen C.A., Selvakumaran J., Notingher I., Jell G., Hench L.L., Stevens M.M. In vitro toxicology evaluation of pharmaceuticals using Raman micro-spectroscopy. J. Cell. Biochem. 2006;99:178–186. doi: 10.1002/jcb.20884. [DOI] [PubMed] [Google Scholar]
- 24.Christopher Jeyaseelan S., Milton Franklin Benial A., Kaviyarasu K. Vibrational, spectroscopic, chemical reactivity, molecular docking and in vitro anticancer activity studies against A549 lung cancer cell lines of 5-Bromo-indole-3-carboxaldehyde. J. Mol. Recogn. 2021;34:1–20. doi: 10.1002/jmr.2873. [DOI] [PubMed] [Google Scholar]
- 25.Prasetyaningrum P.W., Bahtiar A., Hayun H. Synthesis and cytotoxicity evaluation of novel asymmetrical mono-carbonyl analogs of curcumin (AMACs) against vero, HeLa, and MCF7 cell lines. Sci. Pharm. 2018;86 doi: 10.3390/scipharm86020025. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.