Abstract
Purpose:
Immune checkpoint blockade (ICB) agents and adoptive cell transfer (ACT) of tumor infiltrating lymphocytes (TIL) are prominent immunotherapies used for the treatment of advanced melanoma. Both therapies rely on activation of lymphocytes that target shared tumor antigens or neoantigens. Recent analysis of patients with metastatic melanoma who underwent treatment with TIL ACT at the National Cancer Institute (NCI) demonstrated decreased responses in patients previously treated with anti PD-1 agents. We aimed to find a basis for the difference in response rates between anti PD-1 naïve and experienced patients.
Experimental Design:
We examined the tumor mutational burden (TMB) of resected tumors and the repertoire of neoantigens targeted by autologous TIL in a cohort of 112 anti PD-1 naïve and 69 anti PD-1 experienced patients.
Results:
Anti-PD-1 naïve patients were found to possess tumors with higher TMBs (352.0 vs. 213.5, p = 0.005) and received TIL reactive with more neoantigens (2 vs. 1, p = 0.003) compared to anti-PD-1 experienced patients. Among patients treated with TIL ACT, TMB and number of neoantigens identified were higher in ACT responders than ACT non-responders in both anti-PD-1naïve and experienced patients. Among patients with comparable TMBs and predicted neoantigen loads, treatment products administered to anti-PD-1 naïve patients were more likely to contain T-cells reactive against neoantigens than treatment products for anti-PD-1 experienced patients (2.5 vs. 1, p = 0.02).
Conclusions:
These results indicate that decreases in TMB and targeted neoantigens partially account for the difference in response to ACT and that additional factors likely influence responses in these patients.
Introduction:
In the past decade immunotherapy has revolutionized the treatment landscape and improved survival for patients with multiple cancer types. SEER data for metastatic melanoma showed a 5-year survival of 16% from 2003–2009 compared to 27.3% from 2010–2016 [1, 2]. Treatment guidelines have evolved in parallel with landmark clinical trials using adoptive cell transfer (ACT) or immune checkpoint blockade (ICB) agents. Treatment with ICB has received FDA approval as a first line therapy for the treatment of metastatic melanoma [3–7]. Notably, 5-year survival rates as high as 52% have been reported in response to combination ICB utilizing anti CTLA-4 and PD-1 antibodies [7]. Nevertheless, effective therapies are needed for up to two-thirds of patients treated with ICB whose tumors either do not respond or recur after an initial response.
ACT, a form of cancer immunotherapy in which TILs harvested from a patient’s tumor are rapidly expanded in vitro and administered back to the patient after a lymphodepleting regimen, represents an alternative approach to treat patients with metastatic melanoma. Pilot studies demonstrating the effectiveness of TIL ACT for patients with metastatic melanoma were first published in 1988 and subsequently improved [8–13]. The standardization of a preparative nonmyeloablative lymphodepleting chemotherapy regimen subsequently enhanced the efficacy illustrated in earlier ACT iterations [10–12] [14]. Additional clinical trials across multiple centers substantiated the therapeutic potential of ACT for metastatic melanoma, with objective responses ranging from 38–72% and complete responses reported in 8–24% of treated patients [13, 15–17].
Within clinical trials evaluating TIL therapy, retrospective analyses illustrated no difference in response between patients who had previously received anti CTLA-4 therapy and those who had not [13, 16, 17]. While a global Phase 2 study showed that patients with anti PD-1 experienced stage IIIC/IV melanoma can respond to TIL therapy, with an overall response rate (ORR) of 38% with two (4%) complete responders, the efficacy was not compared to that of an anti PD-1 naïve population [18]. A comprehensive analysis of the melanoma TIL experience at the National Cancer Institute (NCI) Surgery Branch has demonstrated decreased responses to TIL in patients previously treated with anti PD-1 agents [19]. In a cohort of 192 patients with metastatic melanoma who were treated with autologous TIL, the ORR was 56% in anti PD-1 naïve patients compared to 24% in anti PD-1 experienced patients. While ACT is a promising therapy, the ubiquity of ICB in the treatment of metastatic melanoma raises the question of whether ACT can be used as a salvage therapy in ICB-experienced patients. The factors responsible for the decreased efficacy of TIL in the setting of anti PD-1 experienced disease need to be identified in order to improve TIL therapy for these patients.
The functional targets of both TIL and immune checkpoint blockade appear to be tumor neoantigens [20–29] and tumor mutational burden (TMB) has been positively correlated with response to immunotherapy in non-small cell lung cancer, mismatch repair deficient colorectal cancers, and melanoma [28–34]. We aimed to determine if TMB and the number of neoantigens identified accounted for the difference in response rates to TIL between anti PD-1 naïve and experienced patients. Our current study is the first comprehensive effort at neoantigen identification in patients with metastatic melanoma who were treated with ACT and demonstrated that TIL recognition of tumor neoantigens was higher in anti PD-1 naïve patients compared to experienced patients. Furthermore, TMB and TIL recognition of tumor neoantigens were shown to be associated with the likelihood of clinical response to treatment with autologous TIL in both anti PD-1 naïve and experienced patients.
Materials and Methods
Generation of ACT Product
All patients were enrolled on protocol NCT00068003 for the purpose of TIL harvest and were required to have histologically proven metastatic melanoma with measurable target lesions ≥ 1 cm on cross-sectional imaging, clear progression of disease, and ≥ 4 weeks without therapy. Patients were required to be ≥ 18 years of age with adequate hepatic, renal, and bone marrow function, an Eastern Cooperative Oncology Group (ECOG) performance status < 2, and a life expectancy of > 3 months. Exclusion criteria included contraindications to IL-2 administration and any autoimmune disease that required immunosuppressive medications. Melanoma metastases were surgically resected based on the size of the deposit and ease of resectability. Tumors were processed for TIL as previously described [35, 36] and infusion products were generated by rapid expansion of T-cells with irradiated PBMC feeder cells, anti-CD3 antibody, and IL-2 [37].
Treatment Protocols
Following the successful development of an ACT product, patients were enrolled on and provided written informed consent for one of three TIL treatment protocols approved by the Institutional Review Board of the NCI (NCT01319565, NCT01993719, or NCT02621021). Additional exclusion criteria for NCI01319565 included contraindications to TBI. Patients underwent preparative lymphodepleting regimens starting on day −7 consisting of cyclophosphamide 60 mg/kg/day for 2 days and concomitant fludarabine 25 mg/m2/day for 5 days. Patients randomized to the TBI arm of NCT10319565 received 1200 cGy TBI over a 3-day period beginning on the last day of fludarabine administration (day −3). Patients randomized to receive pembrolizumab on NCT02621021 received 2 mg/kg pembrolizumab on day −2 and every 3 weeks after ACT treatment for a total of 4 doses. Patients received an intravenous infusion of autologous TIL on day 0 followed by aldesleukin at 720,000 IU/kg every 8 hours to physiologic tolerance.
Response to treatment was assessed using Response Evaluation Criteria in Solid Tumors (RECIST) version 1.0 with the first set of cross-sectional imaging obtained 4 weeks after treatment [10–14, 17].
Tumor Whole Exome Sequencing and HLA Determination
Whole exome sequencing of patients’ tumor and normal peripheral blood cells, transcriptome analysis of patients’ tumors [38] and RNA-seq gene expression analysis [39] was carried out as previously described. For 8 of the 73 samples that were screened for neoantigen responses, 3618, 3625, 3633, 3652, 3873, 4169, 4178 and 4296, RNA-seq data was not available due to insufficient material or degraded RNA. For these samples, the mean expression of the corresponding transcripts was determined for a transcriptome database generated from melanomas sequenced in the Surgery Branch and fragments per kilobase of transcript per million mapped reads (FPKM) were determined using Cufflinks under default settings [40]. All samples’ FPKM values were then normalized using pythons sklearn.RobustScaler package. For expressed genes the median value across all samples was used to determine decile cutoffs and then all genes were binned into the appropriate deciles. All genes with FPKM values of 0 were grouped into decile 1 for the composite RNA-expression.
HLA typing was performed for all patients using two HLA prediction algorithms, PHLAT [41] and HLA-LA [42]. Four-digit resolution of HLA alleles was determined by identifying consensus calls made from patients’ WES data obtained for each locus. All HLA predictions were compared with the results of patients’ 2-digit resolution as determined by the NIH HLA Laboratory and are reported in Table S1.
CD8+ TIL Density
Melanoma specimens were previously stained with CD8 immunohistochemical (IHC) stains (Clone SP57, Roche, Cat#790-4460) at the NCI Center for Cancer Research, Laboratory of Pathology. IHC slides were scanned using a Hmamatsu NanoZoomer S210 Digital slide scanner: C13239–01. Tumor center areas were annotated by a board-certified pathologist (B.G) using QuPath Software v0.3.0. CD8 density (cells/mm2) in tumor center areas was calculated using the “Positive cell detection” function in QuPath.
Neoantigen Identification
Initial Screening
All patient samples underwent screening with tandem minigenes (TMGs) and additional screening with corresponding peptide pools was performed for 95% of anti PD-1 naïve patients and 97% of anti PD-1 experienced patients, as previously described [38] [43]. TMGs were formed by concatenated minigenes designed as 25mers centered around mutated amino acids and synthesized in pcRNA6SL plasmid (Genscript, NJ) for in vitro RNA transcription (mMESSENGER IVT Kit, Thermo Fisher Scientific, USA). Each TMG contained 15–20 minigenes and the number of TMGs per patient ranged from 2–18 depending on the number of vetted variants and variants chosen for screening. For tumors with TMB greater than 200, transcriptome analysis was used to limit the number of variants screened to between 184 and 324. For two patients, 3602 and 3735, additional variants were screened based on predictions generated by a recently published algorithm [39], leading to the identification of two additional neoantigens, RINT1 and TMG12 respectively.
Autologous patient-derived immature dendritic cells [15] or CD40L-activated B-cells were cultured as previously described [38, 43]. Antigen presenting cells (APC) were then transfected with TMG RNA and rested overnight. In earlier experiments transfection was performed via electroporation (BTX-830) and later by Lipofectamine™ MessengerMAX™ transfection reagent (Thermo Fisher Scientific, USA). The two methods of transfection were compared side-by-side before transitioning permanently to the transfection reagent.
Alternatively, peptides corresponding to the TMGs were synthesized either commercially or in-house by the Surgery Branch peptide synthesis core via Fmoc chemistry. Peptides were dissolved in dimethyl sulfoxide (DMSO) and combined in equal concentrations to form peptide pools containing 8–20 peptides. Dendritic cells were pulsed with peptide at a concentration of 10 μg/mL for long peptides or 1 μg/mL for minimal peptides and incubated for 2 hours at 37°C.
Patient infusion products were thawed, rested overnight in TIL media (1:1 AIM V and RPMI, 5% human serum, 2 mM L-glutamine, 25 mM HEPES, 10 μg/mL gentamycin, 100 U/mL penicillin, and 100 μg/mL streptomycin) with IL-2 (3,000 IU/mL), and co-cultured with transfected APCs the following day. Both APCs and TIL were washed prior to co-culture. Lymphocyte recognition of candidate neoantigens was evaluated via IFN-γ release by ELISpot and by upregulation of 4–1BB by flow cytometry.
Determination of HLA Restriction
COS7 cells were transfected with patient-specific class I HLA DNA plasmids (100 ng/well) and TMG plasmids positive on initial screening (100 ng/well) using Lipofectamine 2000 (Thermo Fisher Scientific, USA). Co-culture with patient infusion product was performed the following day, as described above. Both COS7 and TIL cells were washed prior to co-culture.
Identification of Minimal Peptide
Highly predicted minimal peptides (8–10mers) corresponding to positive TMGs were synthesized either commercially or in-house, pulsed onto dendritic cells or COS7 if the restriction was previously determined, and co-cultured with infusion product as described above. If a specific mutant peptide was able to be identified, specificity for the mutant peptide was demonstrated by the lack of recognition of the corresponding wild type peptide or preferential recognition of the mutant peptide in titration experiments.
Statistical Analysis
Continuous variables were compared with nonparametric Mann-Whitney U test. Categorical variables were compared using Fisher’s exact test for 2 groups or Chi-square test when the number of groups was greater than 2. Ordered categorical variables such as age and stage were compared using the two-tailed exact Cochran-Armitage trend test. P-values were not adjusted for multiple comparisons. All statistical analyses were performed using GraphPad software. This work used the computational resources of the NIH HPC Biowulf cluster (http://hpc.nih.gov).
Study Approval
All patients signed informed consents approved by the Institutional Review Board of the NCI in accordance with the principles of the Declaration of Helsinki.
Data Availability
The data generated in this study are publicly available in the database of Genotypes and Phenotypes (dbGaP) at phs001003.v2.p1 and within the article and its supplementary data files.
Results:
Patient Characteristics
We evaluated potential correlates of response to TIL ACT with a study population comprised of 181 patients with metastatic melanoma who underwent tumor harvest for TIL treatment at the National Cancer Institute (NCI) between 2011 and 2018 (CONSORT diagram in Figure 1). There is significant overlap between the patients included in this cohort and the cohort reported in Setter et al. [19]. The difference in the cohorts arises from the exclusion of patients treated on earlier protocols, as they were subject to more variability in their lymphodepletion regimens and were less likely to have available tumor and infusion product for analysis, and the inclusion of patients who received pembrolizumab with their TIL therapy. In this group, 112 patients were anti PD-1 naïve, and 69 patients were anti PD-1 experienced (Table 1).
Figure 1. Consort diagram for patients who underwent TIL harvest with intent to treat on three consecutive TIL ACT treatment protocols.
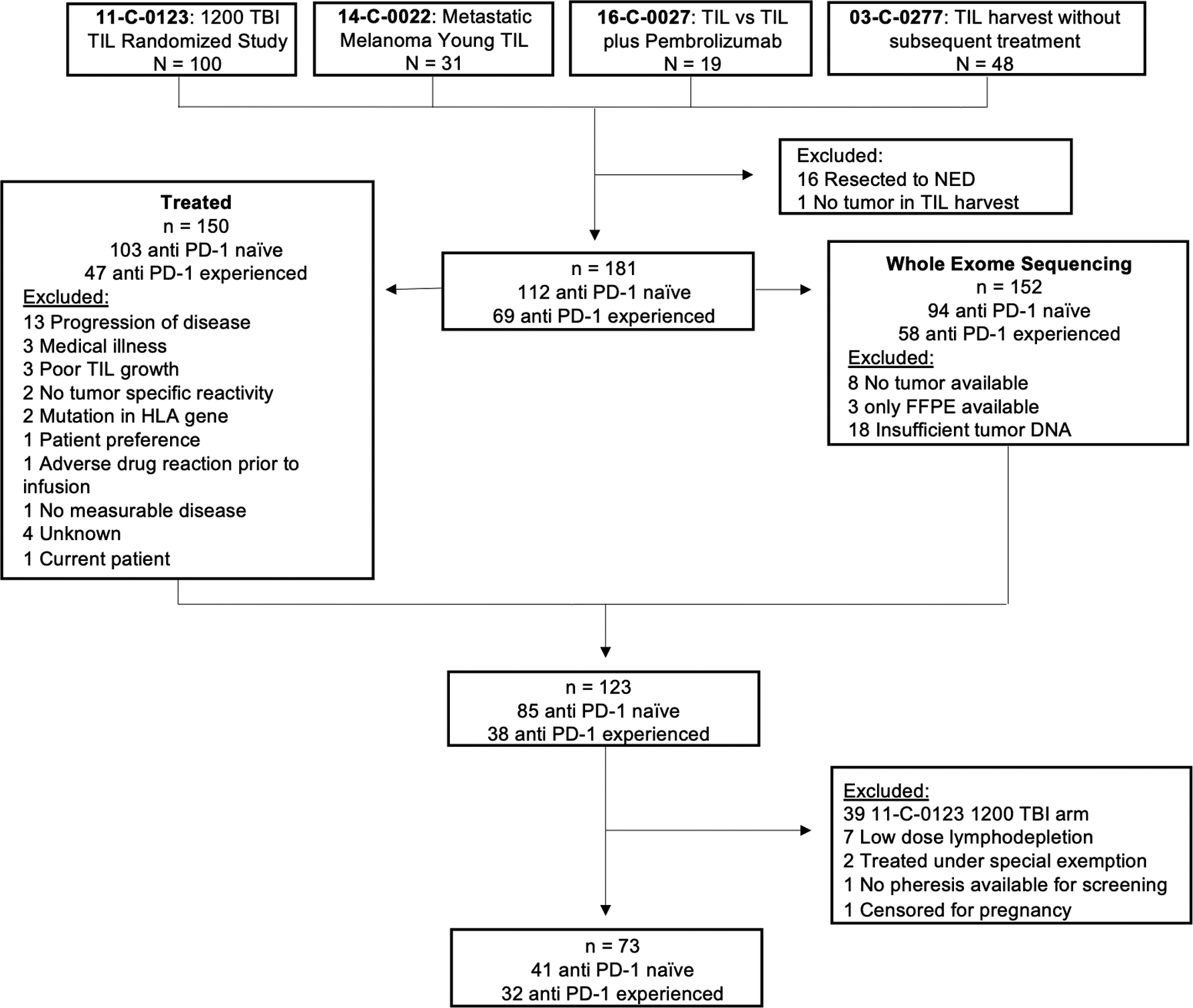
Patients who were treated on earlier protocols were excluded given more variability in the lymphodepletion regimen and less availability of tumor and infusion product. Patients who received pembrolizumab as part of the treatment regimen were included in the cohort.
Table 1.
Patient and Tumor Characteristics
| Characteristic | Anti PD-1 Naïve n (%) |
Anti PD-1 Experienced n (%) |
p |
|---|---|---|---|
| Patients | 112 | 69 | |
| Sex | 0.88 | ||
| Female | 42 (38) | 25 (36) | |
| Male | 70 (62) | 44 (64) | |
| Age, years | |||
| Median | 47 | 46 | 0.33 |
| 18–30 | 14 (12) | 9 (13) | 0.53 |
| 31–45 | 31 (28) | 24 (35) | |
| 46–60 | 62 (55) | 21 (30) | |
| 61–75 | 5 (5) | 15 (22) | |
| Stage, AJCC 8th Edition | 0.87 | ||
| M1a | 20 (18) | 15 (22) | |
| M1b | 25 (22) | 10 (14) | |
| M1c | 49 (44) | 35 (51) | |
| M1d | 18 (16) | 9 (13) | |
| Primary Site of Disease* | 0.81 | ||
| Head/Neck | 23 (21) | 13 (19) | |
| Trunk | 33 (29) | 26 (38) | |
| Extremity | 34 (30) | 17 (25) | |
| Acral | 8 (7) | 6 (9) | |
| Unknown | 14 (12) | 8 (12) | |
| Prior Systemic Therapy | |||
| Anti CTLA-4 | 35 (31) | 64 (93) | <0.0001 |
| Anti BRAF/MEK§ | 7 (13) | 20 (71) | <0.0001 |
| HD IL-2 | 35 (31) | 20 (43) | 0.87 |
| IFN-α | 34 (30) | 24 (35) | 0.62 |
| Chemotherapy | 16 (14) | 15 (13) | 0.22 |
| Other | 21 (19) | 23 (33) | 0.03 |
| BRAF V600 Status‡ | 0.54 | ||
| Mutated | 55 (49) | 30 (43) | |
| Wild Type | 57 (51) | 38 (55) | |
| Year of TIL Treatment† | <0.0001 | ||
| 2011–2014 | 98 (95) | 13 (28) | |
| 2015–2018 | 5 (5) | 34 (72) |
Single patient in anti PD-1 experienced group with two categorically different primaries
Percentages shown out of patients with melanoma harboring BRAF mutations
Single patient without available tumor DNA for sequencing
Percentages shown out of patients who were treated with TIL
Previous Treatments
Patients in the anti PD-1 experienced cohort were more heavily pre-treated than patients in the anti PD-1 naïve cohort. In the anti PD-1 experienced cohort, 93% of patients had previously received anti CTLA-4 ICB compared to 31% of patients in the naïve cohort (p < 0.0001). For patients with tumors harboring BRAF mutations, which was determined by whole exome sequencing (WES), 71% (20/28) of patients in the anti PD-1 experienced cohort had received prior BRAF/MEK inhibition compared to 13% (7/52) of patients in the anti PD-1 naïve group (p < 0.0001). Patients in the anti PD-1 experienced group also had more exposure to additional therapies that included ACT, talimogene laherparepvec (T-VEC), isolated limb perfusion, mTOR inhibitors, GM-CSF injections, vaccines, interleukins other than IL-2, and other monoclonal antibodies (33% vs. 19%, p = 0.03). Only one patient in the anti PD-1 experienced group had no other prior therapies while 25 patients in the anti PD-1 naïve group were completely treatment naïve (p < 0.0001). Lastly, anti PD-1 naïve patients were much more likely to be treated before 2015 while anti PD-1 experienced patients were largely treated in or after 2015, reflecting the time at which anti PD-1 therapy became widely available.
Response to ACT and Effect of Prior ICB
Response to ACT
In the study group of 181 patients, 150 patients received lymphodepletion followed by the adoptive transfer of autologous TIL. Patient and tumor characteristics of patients who underwent treatment is reflective of the larger cohort (Table S2). Analysis of this group revealed that 55% of the 103 anti PD-1 naïve patients achieved an OR, whereas only 26% of the 47 anti PD-1 experienced patients achieved an OR (p = 0.0007) (Figure 2A), consistent with the previously reported data [19]. The nature of the objective responses also differed greatly between the two cohorts as 29 of the 57 (51%) ACT responders in the anti PD-1 naïve group demonstrated complete responses whereas only 1 of the 12 (8%) ACT responders in the anti PD-1 experienced group had a complete response (p = 0.007) (Table S3, Figure 2B).
Figure 2. Effect of prior ICB, BRAF mutational status, and anti BRAF therapy on efficacy of TIL therapy for metastatic melanoma.
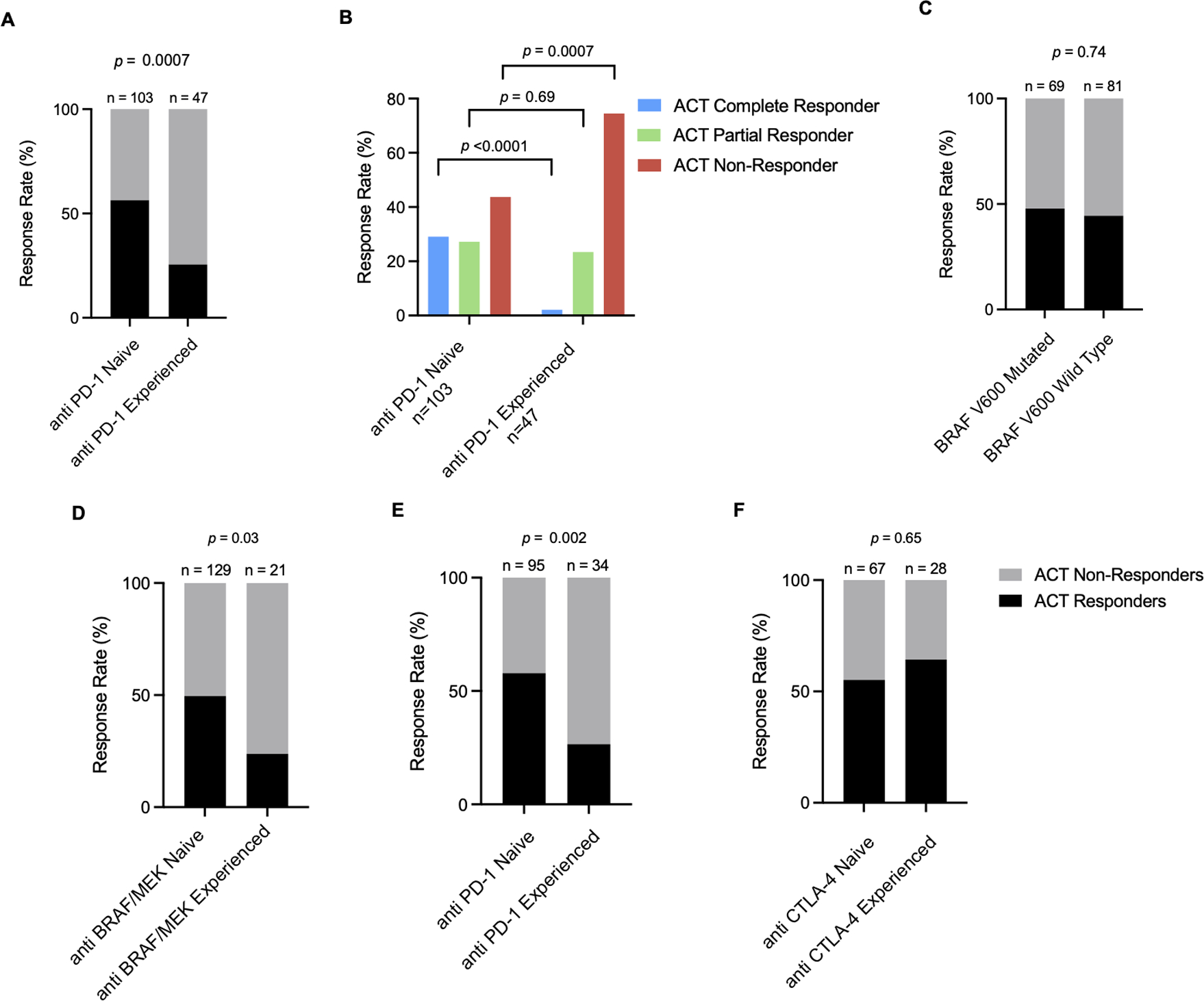
(A) Objective response rates and (B) characterization of objective responses to TIL among anti PD-1 naïve and experienced cohorts. (C) Objective response rates in BRAF V600 mutated and wild type populations. (D) Effect of anti BRAF/MEK on response to TIL ACT. Within the anti BRAF/MEK experienced group, 13 of 21 (62%) patients had also previously been treated with anti PD-1 ICB. (E) Effect of anti PD-1 ICB on response to TIL ACT in an anti BRAF/MEK naïve cohort. (F) Effect of CTLA-4 ICB on response to TIL ACT in an anti PD-1 and anti BRAF/MEK naïve population. Significance values were determined by Fisher’s exact test.
Effect of Previous Treatments on Response to ACT
While BRAF valine 600 (V600) mutational status did not affect the ORR to ACT (Figure 2C), prior treatment with BRAF/MEK inhibitors was associated with a lower response rate to TIL therapy (Figure 2D), as previously noted [19]. For patients with tumors harboring a BRAF mutation, a much higher percentage of patients in the anti PD-1 experienced group received BRAF/MEK inhibitors, compared to the anti PD-1 naïve group (Table 1), which reflects the fact that neither of these treatments were widely available when patients were initially treated in the TIL ACT protocols. Nevertheless, evaluation of the 129 patients treated with TIL ACT who had not received prior BRAF/MEK inhibitors showed that anti PD-1 therapy had an independent effect on response rates to TIL ACT (Figure 2E). In contrast, in patients who had not previously received anti PD-1 or anti BRAF/MEK agents, exposure to prior anti CTLA-4 therapy did not appear to influence response to TIL ACT (Figure 2F).
Tumor Mutational Burden
Whole exome sequencing was carried out on patient’s fresh frozen tumor samples and matching normal peripheral blood cells to compare the TMB between anti PD-1 naïve and experienced cohorts, and to determine the influence of TMB on response to TIL within each group. For 29 of the 181 patients in the study group, fresh frozen tumor samples were either not available, did not yield sufficient DNA, or had low tumor purity, 18 of which were in the anti PD-1 naïve group and 11 of which were in the anti PD-1 experienced group (p > 0.99). Patient and tumor characteristics of the remaining 152 patients with adequate WES data reflect the characteristics of the larger cohort (Table S4), and the compositions of complete, partial, and non-responders to ACT in both the anti PD-1 naïve and experienced groups were also reflective of the larger cohort (p = 0.67 and p = 0.96, respectively).
Effect of TMB on Response to ACT
The median TMB in the anti PD-1 naïve group was higher than that of the anti PD-1 experienced group (352.0 vs. 213.5, p = 0.005) (Figure 3A), and an evaluation of patients who were naïve to prior BRAF/MEK inhibition yielded similar results (358.0 vs. 169.0, p = 0.0003) (Figure 3B). The impact of TMB on response to ACT was further evaluated in the 123 patients who were treated with TIL ACT therapy and had adequate WES data. A difference in median TMB persisted between the anti PD-1 naïve and experienced cohorts in patients who underwent treatment with TIL (357 vs. 209, p = 0.01) (Figure 3C). Furthermore, TMB was higher in ACT responders than non-responders in both anti PD-1 naïve (492 vs. 148, p = 0.0001) and experienced patients (398 vs. 161, p = 0.02) (Figure 3D).
Figure 3. Effect of immune checkpoint blockade on tumor mutational burden.
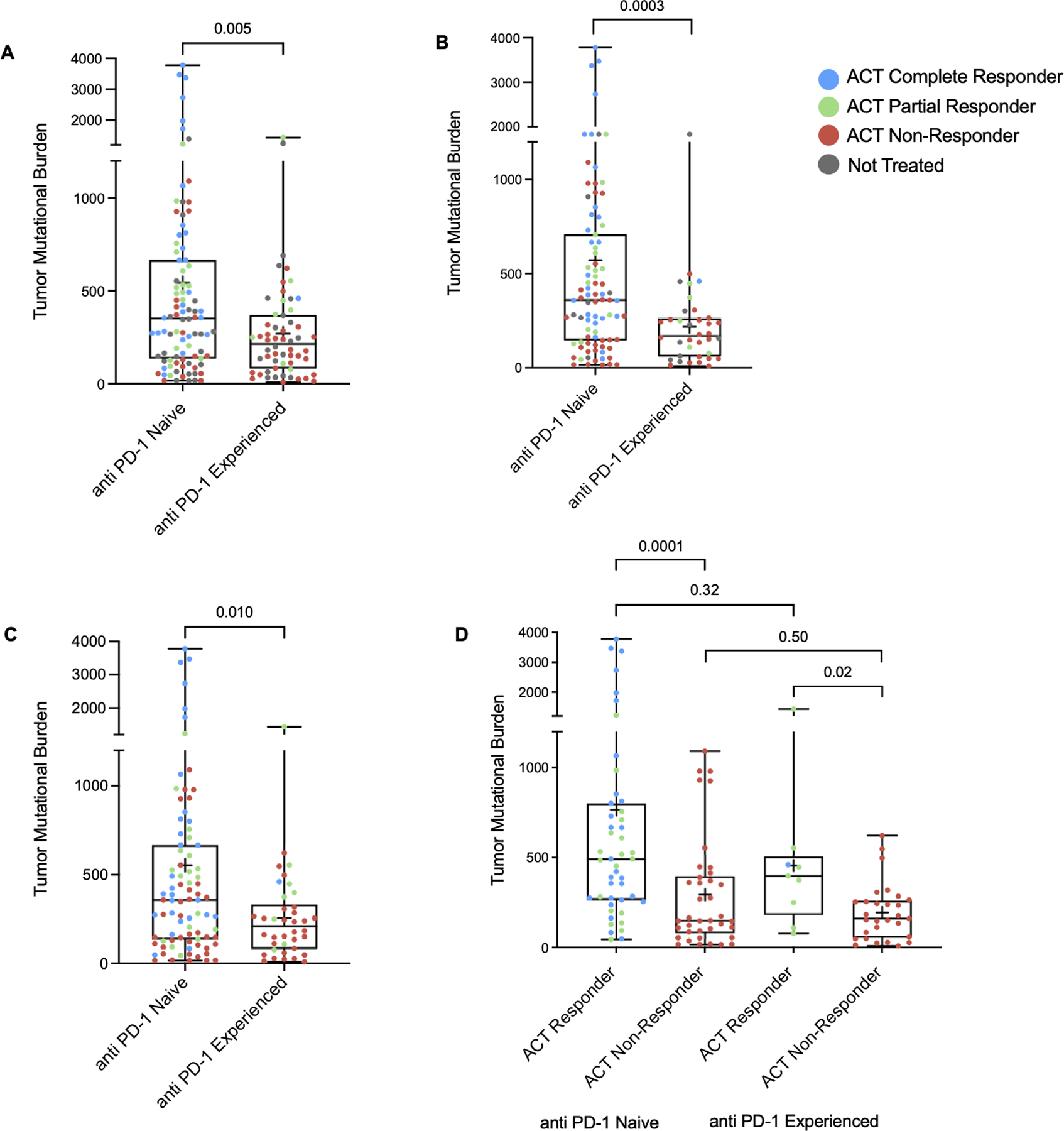
(A) TMB in anti PD-1 naïve and refractory cohorts as a whole, (B) in patients who were naïve to BRAK/MEK inhibition, and (C) in patients who underwent treatment with TIL ACT. (D) TMB within responder and non-responder subgroups in anti PD-1 naïve and refractory cohorts. Significance values were determined by Mann-Whitney U tests.
Neoantigen Identification and TIL Quantification
Samples from 73 patients with adequate WES data who were treated with high dose lymphodepletion without total body irradiation followed by standard autologous TIL were evaluated for neoantigen reactivity. The patient and tumor characteristics of these 73 patients reflects the characteristics of the larger cohort (Table S5). Additionally, the compositions of complete, partial, and non-responders to ACT in both the anti PD-1 naïve and experienced cohorts are similar to the original cohort (p = 0.43 and p = 0.86, respectively). The median TMBs for anti PD-1 naïve and experienced patients were similar to those of the larger cohort (358 vs. 237, p = 0.13).
CD8+ TIL Density
Within the cohort of 73 patients whose infusion products underwent screening for TIL recognition of tumor neoantigens, 49 had melanoma specimens with immunohistochemical (IHC) stains for CD8. The density (cells/mm2) of CD8+ TIL in the tumor center was determined by a board-certified pathologist (B.G.) as described in the methods. There was a slightly higher median CD8+ TIL density in anti PD-1 experienced patients than anti PD-1 naïve patients (107 vs. 34.5, p = 0.06) (Table S5). These findings are consistent with previous reports that PD-1 ICB increases T-cell migration and infiltration into tumors [44] [45].
Characterization of Mutations Identified
A list of the somatic variants identified and screened in this patient cohort is provided in Table S6. The Kyoto Encyclopedia of Genes and Genomes (KEGG) and Gene Ontology (GO) gene sets for antigen processing and presentation were queried to identify mutated genes within these gene sets in our patient cohorts (Table S6). Mutated genes in these gene sets were then analyzed by rare exome variant ensemble learner (REVEL), a tool for predicting the pathogenicity of mutations [46]. A REVEL score of 0.5 was used to determine if a mutation was deleterious or tolerated, with scores above 0.5 corresponding to a sensitivity of 0.754 and specificity of 0.891 [46]. REVEL scores are reported in Table S6. There were 77 mutated genes from 30 patients in the KEGG gene set, of which 58 genes were from 18 anti PD-1 naïve patients and 19 genes were from 12 anti PD-1 experienced patients (p = 0.55 and p = 0.58, respectively), and 240 mutated genes from 51 patients in the GO gene set, of which 179 genes were from 30 anti PD-1 naïve patients and 61 genes were from 21 anti PD-1 experienced patients (p = 0.42 and p = 0.49, respectively). Only three mutated genes from the KEGG gene set were predicted to be deleterious and they were all from the tumor of an anti PD-1 naïve patient with a complete ACT response. From the GO gene set, 49 mutated genes from 28 patients, of which 17 were anti PD-1 naïve and 11 were anti PD-1 experienced, were predicted to be deleterious. There was no difference in response rates between patients with a predicted deleterious GO mutation and those without (46% vs. 33%, p = 0.26).
Comparison of TIL Recognition of Tumor Neoantigens in anti PD-1 Naïve and Experienced Patients
T cell responses against neoantigens were identified in 32 of the 41 (78%) patients in the anti PD-1 naïve group and 17 of the 32 (53%) patients in the anti PD-1 experienced group (p = 0.02) and none of the neoantigens identified were shared between patients (Table S7). The ratio of class I to class II restricted neoantigens identified did not differ between the anti PD-1 naïve and experienced cohorts (p = 0.35) (Figure 4A). The median number of neoantigens identified per patient was two in the anti PD-1 naïve cohort and one in the anti PD-1 experienced cohort (p = 0.003) (Figure 4B) and this difference persisted in an evaluation of patients who were naïve to BRAF/MEK inhibition (p = 0.005) (Figure 4C). In 11 cases, pre-expanded TIL from tumor fragments were screened to determine the specific target of tumor specific T-cells. In all cases where the relevant TIL population was chosen for rapid expansion and inclusion in the infusion product, TIL recognizing tumor neoantigens found in the pre-expanded TIL were also detected in the infusion product.
Figure 4. TIL recognition of tumor neoantigens.
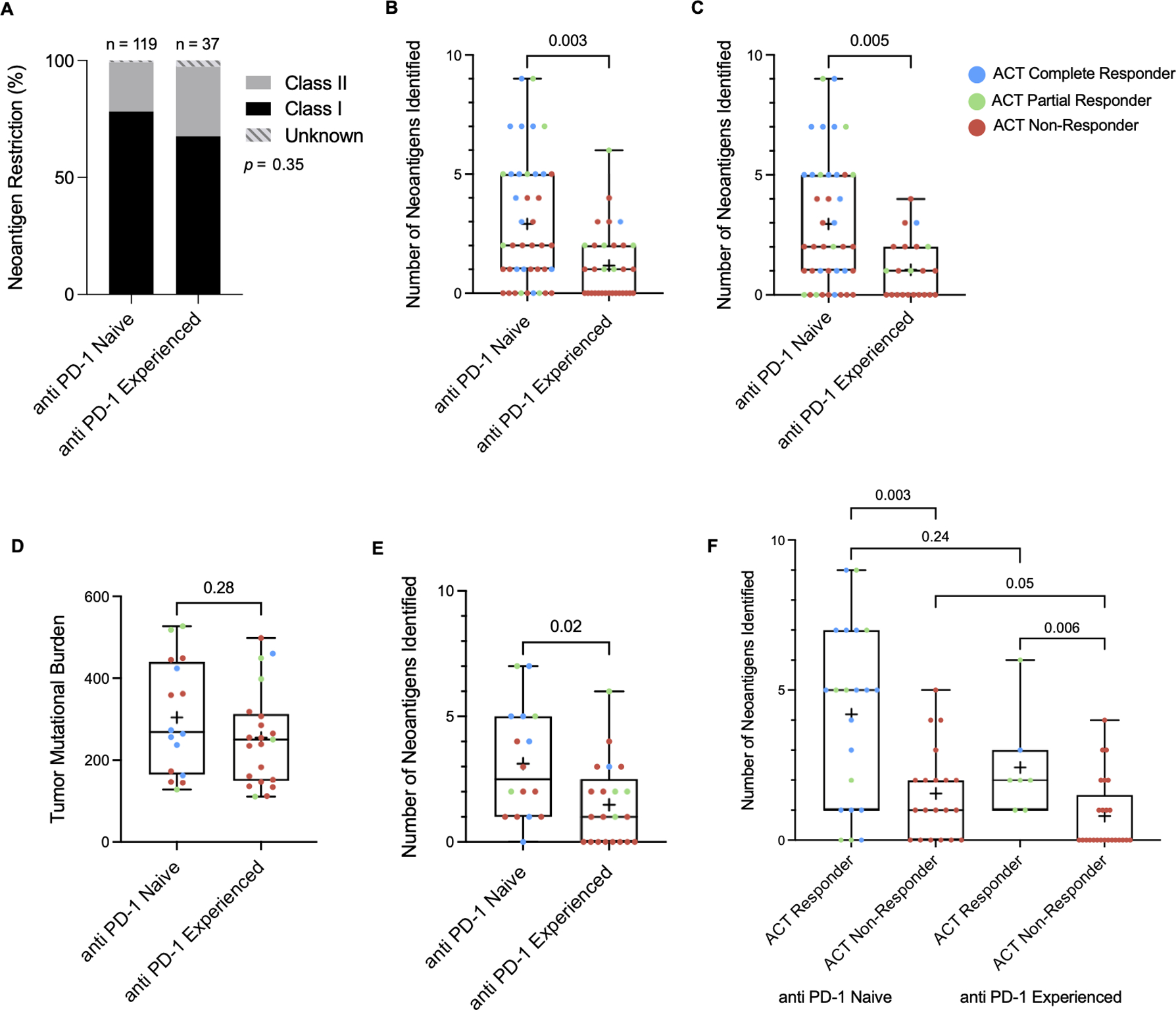
(A) The ratios of class I to class II restricted neoantigens within anti PD-1 naïve and experienced cohorts was found to be similar. (B) Number of neoantigens discovered in anti PD-1 naïve and experienced cohorts as a whole and (C) in patients who were naïve to BRAK/MEK inhibition. (D) TMB in the 2nd and 3rd quartiles illustrate similar TMB between the anti PD-1 naïve and experienced groups with a (E) persistent difference in number of neoantigens identified in the corresponding group of patients. (F) Number of neoantigens identified within ACT responder and non-responder subgroups in anti PD-1 naïve and experienced cohorts. Significance values were determined by Mann-Whitney U and Fisher’s exact tests.
TIL Recognition of Tumor Neoantigens in Patients with Similar TMBs
An attempt was made to determine if TIL recognition of tumor neoantigens differed between the anti PD-1 naïve and experienced cohorts independent of TMB. Analysis of patients bearing tumors with moderate TMBs in the 2nd and 3rd quartiles was performed in order to account for very high TMBs seen in the anti PD-1 naïve cohort as well as the very low TMBs that are outside the normal range seen in the majority of melanoma samples. A similar difference in TIL recognition of tumor neoantigens was found between the anti PD-1 naïve and experienced patients (p = 0.02), despite having similar TMB (p = 0.28) (Figure 4D and 4E).
Correlation Between TIL Recognition of Tumor Neoantigens and ACT Response
Patients who responded to autologous TIL treatment had more neoantigens identified than those who did not respond in both the anti PD-1 naïve and experienced cohorts (naïve: 5 vs. 1, p = 0.003 and experienced: 2 vs. 0 p = 0.006) (Figure 4F). Notably, at least one neoantigen was identified for all patients who achieved a durable response of at least 6 months, except for one patient who was not found to have TIL recognition of tumor neoantigens who was naïve to anti PD-1 therapy and has an ongoing complete response beyond 86 months. Among patients whose infused T-cells recognized at least one neoantigen, the response rates were 56% in the anti PD-1 naïve cohort and 41% in the anti PD-1 experienced cohort (p = 0.32). However, 4 of the 7 ACT responders in the anti PD-1 experienced group had response durations of 5 months or less. When comparing ACT responses of at least 6 months duration, the response rates for patients with at least one neoantigen identified are 56% and 18% for anti PD-1 naïve and experienced patients, respectively (p = 0.009).
Predicted MHC Binding and Expression of Neoantigens
The landscape of potential class I restricted neoantigens was evaluated by using NetMHCpan4.0 affinity algorithm with predicted binding scores, or ranks, of less than 0.5 considered to be indicative of a strong binder (Table S6). There was a slightly higher percentage of strong binders predicted in the anti PD-1 naïve patients compared to the anti PD-1 experienced patients (50% vs. 48%, p = 0.005), but there was no difference in the median rank of potential neoantigens that were predicted to be strong binders (0.1712 vs. 0.1681, p = 0.43). Additionally, there was no difference in the percentage of strong binders predicted between ACT responders and non-responders (50% vs. 50%, p = 0.88). The median rank of potential neoantigens was slightly lower in ACT responders than in ACT non-responders (0.1689 vs. 0.1730, p = 0.02). Of note, 32 of the 156 (20.5%) neoantigens that were identified had predicted netMHC binding ranks greater than 0.5 (Table S7). In addition, all of the identified neoantigens were encoded by gene products that were expressed at a level of 1 or more transcripts per million (TPM), and 78% were expressed at a level of 10 or more TPM (Table S7), demonstrating the strong influence of expression on neoantigen recognition, as previously described [39].
Correlation Between TMB and Neoantigens Identified
Although the number of neoantigens identified was only weakly associated with TMB (p = 0.002, R2 = 0.13), there was a stronger positive relationship seen between the number of neoantigens identified and the number of variants screened (p < 0.001, R2 = 0.29) (Figures 5A and 5B), as screening was limited to approximately 200 variants per patient (see methods). This trend persisted in both the anti PD-1 naïve and experienced groups, although the correlation between the number of neoantigens identified and number of variants screened was stronger in the anti PD-1 naïve cohort (p < 0.001, R2 = 0.43) compared to the experienced cohort (p = 0.06, R2 = 0.12) (Figures 5C and 5D).
Figure 5. Relationship between TMB and TIL recognition of tumor neoantigens.
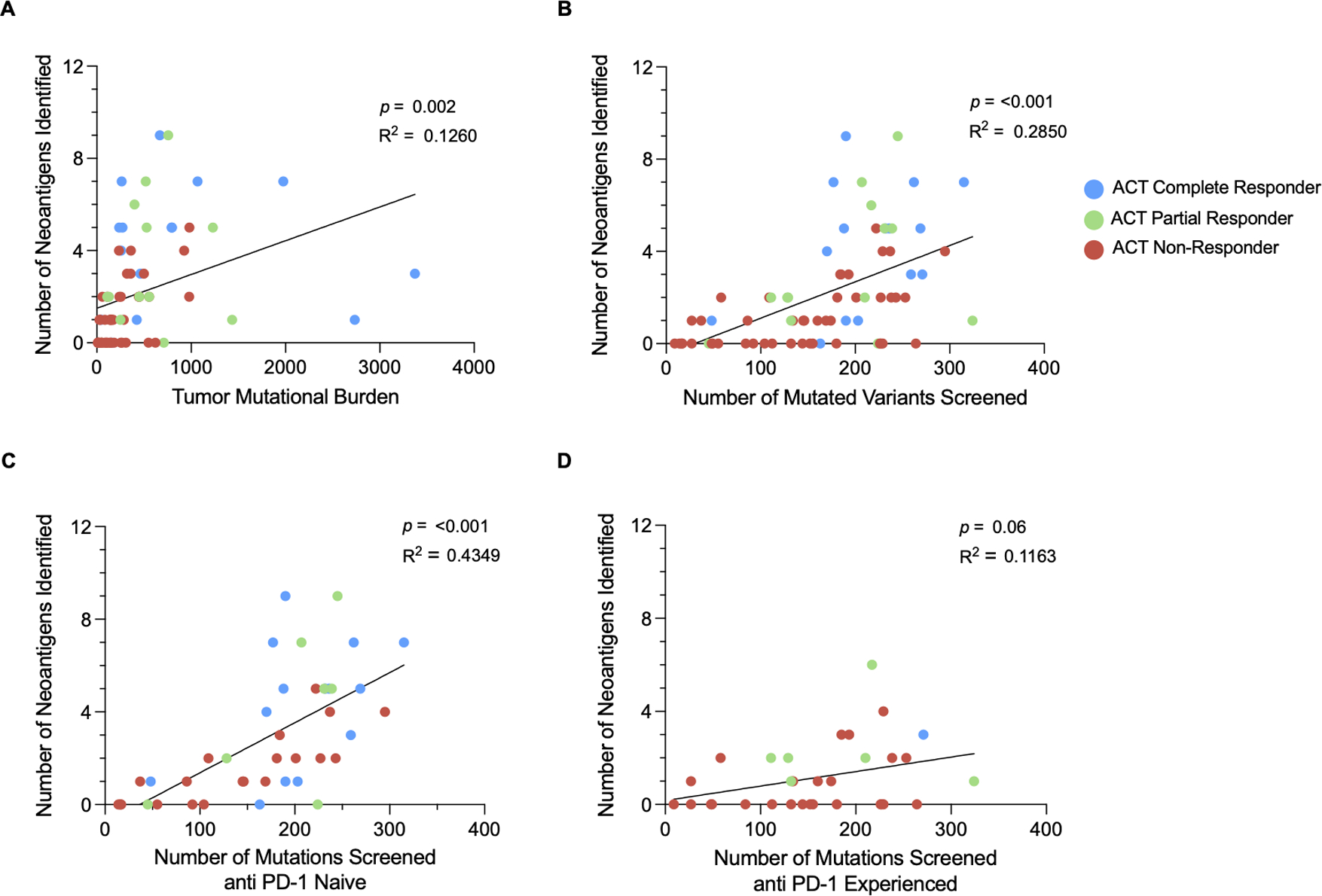
Simple linear regression modeling was used to determine the relationship between the number of neoantigens identified and (A) TMB as well as (B) the number of variants screened. The relationship between neoantigens and variants screened was further analyzed within the (C) anti PD-1 naïve cohort and the (D) anti PD-1 experienced cohort.
Frequency of Neoantigen Detection
The percent of variants screened was higher for the anti PD-1 experienced patients than the anti PD-1 naïve patients (57.0% vs. 30.3%, p <0.001) given the higher TMB in the naïve group and limitations to screening as previously mentioned. To capture as high a frequency of neoantigens as possible, variants with high expression levels, which was highly associated with neoantigen reactivity in a previous study [39], were prioritized for screening. In agreement with previous results, approximately 90% of the neoantigens in both the anti PD-1 naïve and experienced cohorts were encoded by variants that were ranked in the top 5 expression deciles. Given the rate of TIL recognition of tumor neoantigens within this expression range, it was estimated that 90% of class I and II neoantigens were identified for the anti PD-1 experienced cohort while only 60% of class I and 50% of class II neoantigens were identified for the anti PD-1 naïve cohort (Table S8). These findings indicate that the difference in TIL recognition of tumor neoantigens between the anti PD-1 naïve and experienced cohorts may be even more significant than indicated by the results presented here. The frequency of neoantigen reactivity detected was higher in the anti PD-1 naïve group (1.36%) than in the anti PD-1 experienced group (0.73%) (p = 0.0008), and this difference persisted when comparing patients bearing tumors with TMBs in the 2nd and 3rd quartiles where 96% and 94% of variants were screened for the anti PD-1 naïve and experienced patients, respectively (1.28% vs. 0.79%, p = 0.047) (Table S8).
Overall, these results indicate that patients with progressive disease after prior treatment with anti PD-1 blockade had a lower frequency of neoantigen recognition in their cultured TIL samples, which persisted when accounting for differences in TMB, and lower response rates to subsequent TIL ACT.
Discussion
Immune checkpoint blockade agents have changed the treatment paradigm for several advanced stage malignancies. The use of these therapies, especially in combination with one another, is still relatively new and consequent alterations to cancer immunoediting, the tumor microenvironment (TME), and T-cell repertoires are still being described. The study presented here analyzed TMB and characterized neoantigens for a large subset of anti PD-1 naïve and experienced patients treated with TIL ACT at the NIH to investigate factors that may contribute to the difference in ACT response rates between the two patient cohorts.
It is important to note that many of the anti PD-1 naïve patients were treated with TIL ACT at the NIH prior to anti PD-1 blockade being widely available and anti PD-1 experienced patients were typically treated at later dates than their naïve counterparts. However, there were no differences in age or stage at which patients were treated between the two groups.
Similar to previous studies evaluating ICB treatment for metastatic melanoma, we found that TMB was associated with response to TIL therapy [28–30]. Tumors from patients who were previously exposed to anti PD-1 therapy had lower TMB when compared to a PD-1 naïve population, and tumors with very high TMB, which were seen in the anti PD-1 naïve cohort, were not seen in the anti PD-1 experienced cohort. Direct effects of anti PD-1 therapy on TMB would require comparison of tumors before and after exposure to anti PD-1 therapy which was not available for this patient cohort. The difference in TMBs is likely due to selection bias as patients bearing tumors with higher TMB were more likely to demonstrate response to anti PD-1 therapy and therefore would not have been evaluated for TIL therapy at the NIH.
Parallel to the findings for TMB, the neoantigen load was higher in the anti PD-1 naïve cohort compared to the experienced cohort. Likewise, the number of neoantigens identified was higher in ACT responders than in ACT non-responders in both the naïve and experienced cohorts. Importantly, the difference in neoantigen detection between anti PD-1 naïve and experienced patients persisted when evaluating patients with similar TMBs, indicating that there are alternative mechanisms of resistance to TIL. Previous studies have shown a positive correlation between neoantigen load and response but this relationship was based on predicted candidate neoantigens rather than direct in vitro identification of neoantigens as we have performed [31–33, 47]. Our results highlight the inaccuracies of using neoantigen predictions as 20% of the neoantigens that were found had netMHC ranks > 0.5. A recent paper investigated the neoantigen reactive T-cell (NART) diversity in TIL infusion products used for the treatment of patients with metastatic melanoma [48]. This study found that patients with progressive disease had lower NART diversity than patients with stable disease or partial response. However, the paper did not address differences in response or TIL recognition of tumor neoantigens between anti PD-1 naïve and experienced patients. It is important to note that the methods of neoantigen identification in this paper was only for class I restricted T-cells and was limited by the availability of UV exchangeable monomers. Additionally, the lack of TIL recognition of tumor neoantigens in ACT non-responders contrasts with our findings, as we were able to identify several neoantigen reactivities in ACT non-responders, signifying other reasons for disease progression through TIL therapy.
While the predicted peptide-MHC affinity ranks differed slightly between ACT responders and ACT non-responders, there was no difference found between anti PD-1 naïve and experienced patients. Furthermore, the expression levels of all neoantigens found were greater than 1 TPM. Our findings do not account for clonality of the targeted neoantigens which may be affected by prior anti PD-1 therapy and likely plays a role in the effectiveness of an antitumor response [47, 49]. Sub-clonality of neoantigens, which was not examined in this study, may explain why half of the objective responses in the PD-1 experienced group persisted for less than 6 months.
For 33 of the 73 (45%) patients screened for neoantigen reactivity, 22 of which were in the anti PD-1 naïve group, only a fraction of the mutational burden was utilized for neoantigen identification due to limitations of screening to approximately 200 variants per patient. However, the correlation between neoantigen load and the number of variants screened was strong. Notably, the relationship between number of variants screened and neoantigen load was stronger in the naïve cohort than in the experienced cohort, further indicating that there may be a process of immunoediting affecting the number of neoantigens identified in the anti PD-1 experienced cohort.
T-cell differentiation states defined by specific gene enrichments or downregulations, such as TCF7, TIM3, PD-1, CD39, and CD69, have been determined to play a significant role in response to ICB and TIL therapy [50–52]. Furthermore, ICB therapy has been shown to induce distinct changes in gene expression in human T-cells and monocytes [53, 54]. However, the potential role of ICB in altering the phenotype of anti-tumor T-cells remains unclear. Unfavorable changes to the T-cell state could explain the decreased response to TIL as well as the lower neoantigen detection rate in anti PD-1 experienced patients as exhausted T-cells would be unlikely to persist through the rapid expansion used to generate a TIL infusion product. Further analysis of TIL phenotypes from this patient cohort is warranted and if anti PD-1 therapy induces detrimental changes to T-cells it would be beneficial to perform TIL harvest prior to anti PD-1 exposure.
Our findings indicate that TMB and neoantigen load are associated with response to TIL and, while they are partially responsible for the disparity in response between anti PD-1 naïve and experienced patients, additional factors such as T-cell phenotype and tumor heterogeneity likely influence response rates to ACT as well. Additionally, the results indicate that patients without identifiable reactivity against neoantigens are unlikely to respond to TIL, particularly in the anti PD-1 experienced setting, providing support for the contention that an evaluation of neoantigen reactivity should be incorporated into selection criteria for TIL therapy.
Supplementary Material
Translational Relevance.
In patients with advanced melanoma, response to salvage therapies such as adoptive cell transfer (ACT) of tumor infiltrating lymphocytes (TIL) is impacted by previous exposure to anti PD-1 therapy. Identification of factors responsible for these differences is paramount to overcoming them and improving responses to salvage therapies. Our results show a lower detection rate of tumor specific T-cells in anti PD-1 experienced patients’ infusion products despite similar predicted neoantigen loads between anti PD-1 naïve and experienced patients. This difference persists when accounting for higher tumor mutational burdens (TMBs) found in the anti PD-1 naïve cohort. These findings may indicate that tumor specific T-cells from anti PD-1 experienced patients fail to expand during the development of a TIL treatment product, rather than there being a lower neoantigen load in anti PD-1 experienced tumors; this could be overcome by alternative treatments such as ACT with lymphocytes engineered with antitumor T cell receptors (TCRs).
Acknowledgements
We thank Maria Florentin at the NIH for her assistance with peptide synthesis. Funding for this research was provided by the Center for Cancer Research, the intramural division of the NCI and supported by a cooperative research and development agreement (CRADA) with Iovance Biotherapeutics.
Footnotes
The authors declare no potential conflicts of interest.
References
- 1.Howlader N, et al. , SEER Cancer Statistics Review, 1975–2010, National Cancer Institute. Bethesda, MD. 2012. [Google Scholar]
- 2.Howlader N, et al. , SEER Cancer Statistics Review, 1975–2017, National Cancer Institute. Bethesda, MD. 2019. [Google Scholar]
- 3.Hodi FS, et al. , Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med, 2010. 363(8): p. 711–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robert C, et al. , Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med, 2011. 364(26): p. 2517–26. [DOI] [PubMed] [Google Scholar]
- 5.Schachter J, et al. , Pembrolizumab versus ipilimumab for advanced melanoma: final overall survival results of a multicentre, randomised, open-label phase 3 study (KEYNOTE-006). Lancet, 2017. 390(10105): p. 1853–1862. [DOI] [PubMed] [Google Scholar]
- 6.Larkin J, et al. , Overall Survival in Patients With Advanced Melanoma Who Received Nivolumab Versus Investigator’s Choice Chemotherapy in CheckMate 037: A Randomized, Controlled, Open-Label Phase III Trial. J Clin Oncol, 2018. 36(4): p. 383–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Larkin J, et al. , Five-Year Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N Engl J Med, 2019. 381(16): p. 1535–1546. [DOI] [PubMed] [Google Scholar]
- 8.Rosenberg SA, et al. , Use of tumor-infiltrating lymphocytes and interleukin-2 in the immunotherapy of patients with metastatic melanoma. A preliminary report. N Engl J Med, 1988. 319(25): p. 1676–80. [DOI] [PubMed] [Google Scholar]
- 9.Kradin RL, et al. , Tumour-infiltrating lymphocytes and interleukin-2 in treatment of advanced cancer. Lancet, 1989. 1(8638): p. 577–80. [DOI] [PubMed] [Google Scholar]
- 10.Dudley ME, et al. , A phase I study of nonmyeloablative chemotherapy and adoptive transfer of autologous tumor antigen-specific T lymphocytes in patients with metastatic melanoma. J Immunother, 2002. 25(3): p. 243–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dudley ME, et al. , Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J Clin Oncol, 2005. 23(10): p. 2346–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosenberg SA, et al. , Treatment of patients with metastatic melanoma with autologous tumor-infiltrating lymphocytes and interleukin 2. J Natl Cancer Inst, 1994. 86(15): p. 1159–66. [DOI] [PubMed] [Google Scholar]
- 13.Rosenberg SA, et al. , Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin Cancer Res, 2011. 17(13): p. 4550–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dudley ME, et al. , Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. J Clin Oncol, 2008. 26(32): p. 5233–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pilon-Thomas S, et al. , Efficacy of adoptive cell transfer of tumor-infiltrating lymphocytes after lymphopenia induction for metastatic melanoma. J Immunother, 2012. 35(8): p. 615–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Besser MJ, et al. , Adoptive transfer of tumor-infiltrating lymphocytes in patients with metastatic melanoma: intent-to-treat analysis and efficacy after failure to prior immunotherapies. Clin Cancer Res, 2013. 19(17): p. 4792–800. [DOI] [PubMed] [Google Scholar]
- 17.Goff SL, et al. , Randomized, Prospective Evaluation Comparing Intensity of Lymphodepletion Before Adoptive Transfer of Tumor-Infiltrating Lymphocytes for Patients With Metastatic Melanoma. J Clin Oncol, 2016. 34(20): p. 2389–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sarnaik A, et al. , Safety and efficacy of cryopreserved autologous tumor infiltrating lymphocyte therapy (LN-144, lifileucel) in advanced metastatic melanoma patients who progressed on multiple prior therapies including anti-PD-1. Journal of Clinical Oncology, 2019. 37(15_suppl): p. 2518–2518.31154919 [Google Scholar]
- 19.Seitter SJ, et al. , Impact of Prior Treatment on the Efficacy of Adoptive Transfer of Tumor-Infiltrating Lymphocytes in Patients with Metastatic Melanoma. Clin Cancer Res, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boon T, Tumor antigens recognized by cytolytic T lymphocytes: present perspectives for specific immunotherapy. Int J Cancer, 1993. 54(2): p. 177–80. [DOI] [PubMed] [Google Scholar]
- 21.Rosenberg SA, The immunotherapy of solid cancers based on cloning the genes encoding tumor-rejection antigens. Annu Rev Med, 1996. 47: p. 481–91. [DOI] [PubMed] [Google Scholar]
- 22.Rosenberg SA, Development of cancer immunotherapies based on identification of the genes encoding cancer regression antigens. J Natl Cancer Inst, 1996. 88(22): p. 1635–44. [DOI] [PubMed] [Google Scholar]
- 23.Boon T and van der Bruggen P, Human tumor antigens recognized by T lymphocytes. J Exp Med, 1996. 183(3): p. 725–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DuPage M, et al. , Expression of tumour-specific antigens underlies cancer immunoediting. Nature, 2012. 482(7385): p. 405–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Robbins PF, et al. , Mining exomic sequencing data to identify mutated antigens recognized by adoptively transferred tumor-reactive T cells. Nat Med, 2013. 19(6): p. 747–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gubin MM, et al. , Checkpoint blockade cancer immunotherapy targets tumour-specific mutant antigens. Nature, 2014. 515(7528): p. 577–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lu YC, et al. , Efficient identification of mutated cancer antigens recognized by T cells associated with durable tumor regressions. Clin Cancer Res, 2014. 20(13): p. 3401–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Snyder A, et al. , Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med, 2014. 371(23): p. 2189–2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Van Allen EM, et al. , Genomic correlates of response to CTLA-4 blockade in metastatic melanoma. Science, 2015. 350(6257): p. 207–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goodman AM, et al. , Tumor Mutational Burden as an Independent Predictor of Response to Immunotherapy in Diverse Cancers. Mol Cancer Ther, 2017. 16(11): p. 2598–2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rizvi NA, et al. , Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science, 2015. 348(6230): p. 124–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Le DT, et al. , PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med, 2015. 372(26): p. 2509–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hellmann MD, et al. , Genomic Features of Response to Combination Immunotherapy in Patients with Advanced Non-Small-Cell Lung Cancer. Cancer Cell, 2018. 33(5): p. 843–852 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hellmann MD, et al. , Nivolumab plus Ipilimumab in Lung Cancer with a High Tumor Mutational Burden. N Engl J Med, 2018. 378(22): p. 2093–2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dudley ME, et al. , Generation of tumor-infiltrating lymphocyte cultures for use in adoptive transfer therapy for melanoma patients. J Immunother, 2003. 26(4): p. 332–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tran KQ, et al. , Minimally cultured tumor-infiltrating lymphocytes display optimal characteristics for adoptive cell therapy. J Immunother, 2008. 31(8): p. 742–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jin J, et al. , Simplified method of the growth of human tumor infiltrating lymphocytes in gas-permeable flasks to numbers needed for patient treatment. J Immunother, 2012. 35(3): p. 283–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Parkhurst MR, et al. , Unique Neoantigens Arise from Somatic Mutations in Patients with Gastrointestinal Cancers. Cancer Discov, 2019. 9(8): p. 1022–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gartner JJ, et al. , A machine learning model for ranking candidate HLA class I neoantigens based on known neoepitopes from multiple human tumor types. Nature Cancer, 2021. 2(5): p. 563–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Trapnell C, et al. , Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat Protoc, 2012. 7(3): p. 562–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dilthey AT, et al. , HLA*LA-HLA typing from linearly projected graph alignments. Bioinformatics, 2019. 35(21): p. 4394–4396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bai Y, Wang D, and Fury W, PHLAT: Inference of High-Resolution HLA Types from RNA and Whole Exome Sequencing. Methods Mol Biol, 2018. 1802: p. 193–201. [DOI] [PubMed] [Google Scholar]
- 43.Zacharakis N, et al. , Immune recognition of somatic mutations leading to complete durable regression in metastatic breast cancer. Nat Med, 2018. 24(6): p. 724–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Peng W, et al. , PD-1 blockade enhances T-cell migration to tumors by elevating IFN-gamma inducible chemokines. Cancer Res, 2012. 72(20): p. 5209–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chalabi M, et al. , Neoadjuvant immunotherapy leads to pathological responses in MMR-proficient and MMR-deficient early-stage colon cancers. Nat Med, 2020. 26(4): p. 566–576. [DOI] [PubMed] [Google Scholar]
- 46.Ioannidis NM, et al. , REVEL: An Ensemble Method for Predicting the Pathogenicity of Rare Missense Variants. Am J Hum Genet, 2016. 99(4): p. 877–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McGranahan N, et al. , Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science, 2016. 351(6280): p. 1463–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kristensen NP, et al. , Neoantigen-reactive CD8+ T cells affect clinical outcome of adoptive transfer with tumor-infiltrating lymphocytes in melanoma. J Clin Invest, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Riaz N, et al. , Tumor and Microenvironment Evolution during Immunotherapy with Nivolumab. Cell, 2017. 171(4): p. 934–949 e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sade-Feldman M, et al. , Defining T Cell States Associated with Response to Checkpoint Immunotherapy in Melanoma. Cell, 2019. 176(1–2): p. 404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Canale FP, et al. , CD39 Expression Defines Cell Exhaustion in Tumor-Infiltrating CD8(+) T Cells. Cancer Res, 2018. 78(1): p. 115–128. [DOI] [PubMed] [Google Scholar]
- 52.Krishna S, et al. , Stem-like CD8 T cells mediate response of adoptive cell immunotherapy against human cancer. Science, 2020. 370(6522): p. 1328–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Das R, et al. , Combination therapy with anti-CTLA-4 and anti-PD-1 leads to distinct immunologic changes in vivo. J Immunol, 2015. 194(3): p. 950–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kurtulus S, et al. , Checkpoint Blockade Immunotherapy Induces Dynamic Changes in PD-1(−)CD8(+) Tumor-Infiltrating T Cells. Immunity, 2019. 50(1): p. 181–194 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data generated in this study are publicly available in the database of Genotypes and Phenotypes (dbGaP) at phs001003.v2.p1 and within the article and its supplementary data files.


