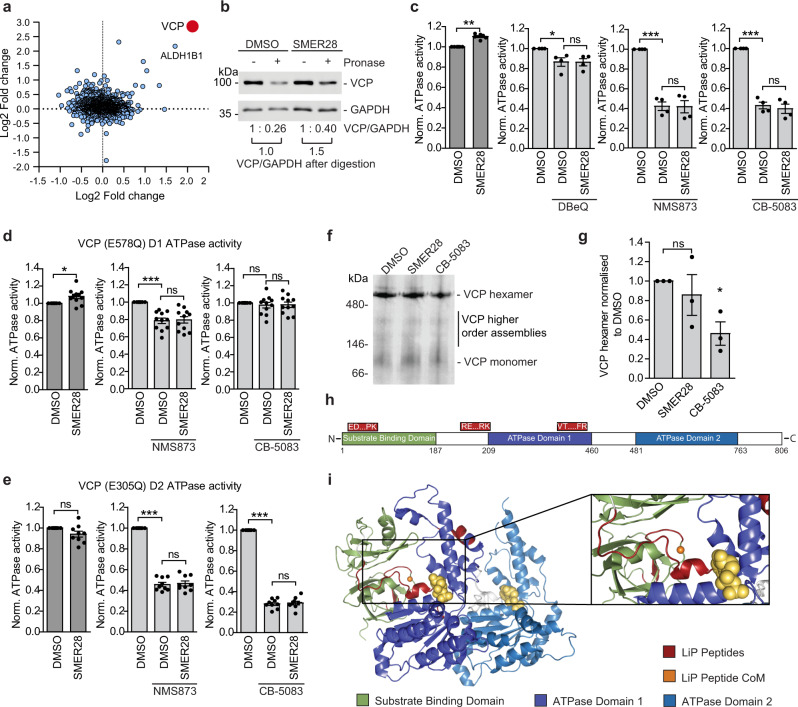
Fig. 2. SMER28 binds VCP and increases VCP D1 ATPase activity.
a SMER28 interactors tested in a reverse competition experiment with free compound at 800 µM. Interactors with a log2-fold-change >2 are labeled in red and were considered significant. Data point size correlates with a number of unique peptides. b VCP levels after pronase digestion in DARTS assay in lysates from HeLa cells treated with 20 μM SMER28 for 1 h before lysis. VCP/GAPDH ratio normalized to non-digested samples and ratio in treated samples compared to DMSO control. c–e in vitro ATPase activity of wild-type VCP (c n = 7), VCP-E578Q mutant (d n = 11), and VCP-E305Q mutant (e n = 9) upon addition of 20 μM SMER28 with and without the addition of VCP inhibitors: 10 μM DBeQ, 10 μM NMS873 or 2 μM CB-5083 after 60 min incubation at 37 °C; statistical analysis of DMSO vs SMER28: one sample t test (c P = 0.0002; d P = 0.0115; e P = 0.0772); statistical analysis of DMSO vs SMER28 in presence of VCP inhibitors: one-way ANOVA with post hoc Tukey test (for details see Source Data file). f, g HeLa cells were treated with 20 µM SMER28 or 5 µM CB-5083 for 6 h, followed by Blue-Native-PAGE electrophoresis; n = 3, quantification in g; one-way ANOVA: P = 0.0489 with post hoc Tukey test. h Linear representation of VCP protein with SMER28 binding peptides from Supplementary Fig. 2i represented on top in red. i Peptides from Supplementary Fig. 2i mapped (red) onto one subunit of VCP with the center of mass (CoM, orange) of the three peptides indicated. Known domains of VCP (green, purple, and blue) and ADP (yellow) are indicated. Bar graphs data presented as normalized mean ± SEM. *P < 0.05, **P < 0.001, ***P < 0.0001; ns not significant. Source data are provided as a Source Data file.