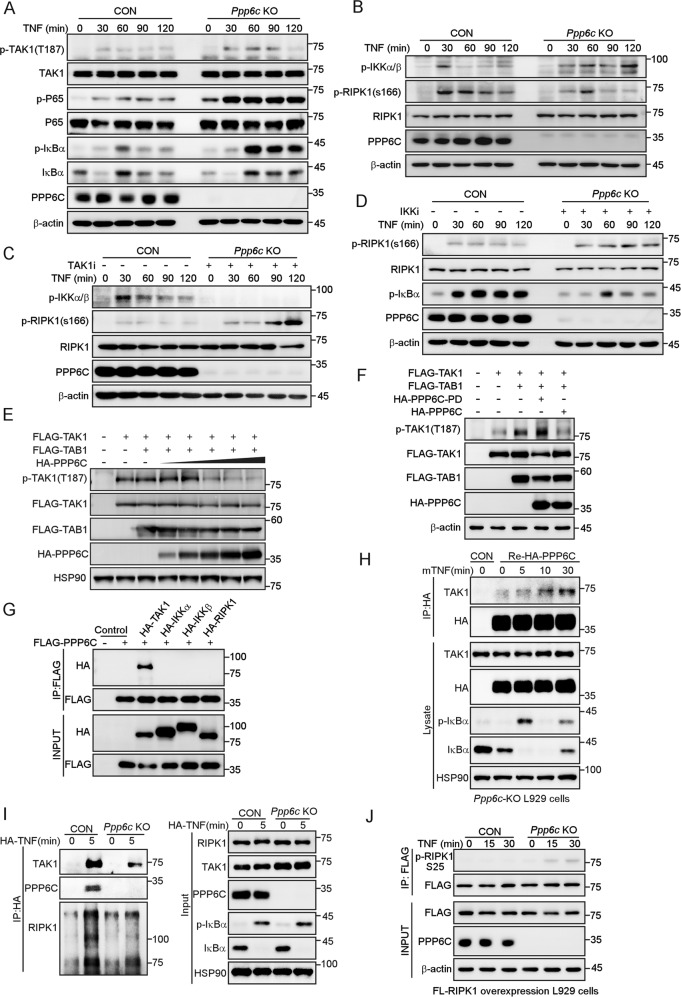
Fig. 4. PPP6C interacts with and dephosphorylates TAK1.
A, B Control and Ppp6c-KO L929 cells were treated with TNF (10 ng/ml) for the indicated time, and then lysed for immunoblot with indicated antibodies. C, D Control and Ppp6c-KO L929 cells were pretreated with TAK1 inhibitor (5Z-7-Oxozeaenol, 1 μM) (C) or IKKα/β inhibitor (IKK16, 1 μM) (D) for 30 min and then treated with TNF (10 ng/ml) for the indicated time. Cell lysates were probed with indicated antibodies. E HEK293T cells were transfected with TAK1 and TAB1 plasmids together with different dose of PPP6C plasmid. Cell lysates were probed with indicated antibodies 24 h after transfection. F HEK293T cells were transfected with TAK1 and TAB1 plasmids together with PPP6C or PPP6C-PD mutant plasmids. Cell lysates were probed with indicated antibodies 24 h after transfection. G HEK293T cells were transfected with the indicated plasmids and then lysed for co-immunoprecipitation assay as indicated. H Ppp6c-KO L929 cells were transduced with HA-PPP6C lentivirus and then treated with TNF (10 ng/ml) for the indicated time, and lysed for co-immunoprecipitation assay as indicated. I Control and Ppp6c-KO L929 cells were stimulated with 1 μg/ml HA-mTNF for 5 min. TNFR1 complex I was immunoprecipitated by anti-HA antibody and analyzed by immunoblotting. J Control and Ppp6c-KO L929 cells were transduced with FLAG-RIPK1 lentivirus and then treated with TNF (10 ng/ml) for the indicated time. FLAG-RIPK1 proteins were immunoprecipitated with anti-FLAG antibody and probed with anti-p-RIPK1 (S25) antibody.