Abstract
Aims
Transthyretin amyloid cardiomyopathy (ATTR‐CM) is a progressive condition caused by deposition of transthyretin amyloid fibrils in the heart and is associated with poor quality of life and a shortened lifespan. This study aimed to describe the prevalence, clinical characteristics, and mortality of patients with ATTR‐CM, using multiple national health registers in Denmark, Finland, Norway, and Sweden.
Methods and results
Transthyretin amyloid cardiomyopathy patients were identified during 2008–2018 using a combination of diagnosis codes for amyloidosis and heart disease and were matched to patients with non‐ATTR heart failure (HF). An identical study design was used in each country to facilitate comparison and aggregation of results. A total of 1930 ATTR‐CM patients were identified from national health registers in the four countries. In 2018, prevalence of ATTR‐CM per 100 000 inhabitants ranged from 1.4 in Denmark to 5.0 in Sweden; a steep increase over time was observed in Sweden and Norway. Median survival from diagnosis was 30 months for ATTR‐CM patients and 67 months for matched HF patients. Survival was significantly lower for female than for male ATTR‐CM patients (median survival: 22 and 36 months), while no significant difference was observed in the HF cohort.
Conclusions
This study provides the first nationwide estimates of the prevalence, clinical characteristics, and mortality of patients with ATTR‐CM, using identical study design across several countries. Findings corroborate previous case series showing high mortality in ATTR‐CM, two‐fold higher than for other HF patients and higher in women than men, highlighting the need for more precise and early diagnosis to reduce the disease burden.
Keywords: ATTR‐CM, Heart failure, Amyloidosis, Prevalence, Mortality, Red flags
Introduction
Transthyretin amyloidosis (ATTR) originates from a misfolding of the precursor protein transthyretin (TTR) mainly produced in the liver. Transthyretin amyloidosis can manifest as cardiomyopathy (ATTR‐CM) when TTR amyloid fibrils are deposited in heart tissue resulting in progressive heart failure and premature death. ATTR‐CM is caused by hereditary mutations in the transthyretin (TTR) gene (variant ATTR or ATTRv) or from a wild‐type allelic constitution (ATTRwt) associated with aging. 1
ATTR‐CM is associated with poor quality of life 2 and half of all ATTR‐CM patients die within 4 years of diagnosis. 3 , 4 , 5 , 6 Despite the severity of the disease, diagnosis is often delayed or ATTR‐CM remains undiagnosed due to the diffuse symptoms of the disease 2 and lack of awareness among healthcare professionals. 1 , 7 One reason for insufficient diagnostic attention to ATTR‐CM in the medical community is lack of reliable knowledge on epidemiology of the disease as most studies have focused on smaller series of patients referred to tertiary care centres.
Lack of awareness and attention and the resulting delay in diagnosis of ATTR‐CM may affect women more than men as prevalence of ATTR‐CM is known to be lower in women compared with men; previous studies estimated approximately 13% of ATTRwt‐CM patients 8 and 22% ATTRv‐CM patients 9 to be female. There are indications that ATTR‐CM may be underdiagnosed even more in women compared with men. 10 , 11 For instance, studies including autopsy for ATTR‐CM diagnosis have found higher proportions of women than studies that diagnose ATTR‐CM with common measures in living patients. 8
Few studies so far have used large register‐based data sets to estimate the prevalence and characteristics of ATTR‐CM 12 , 13 , 14 and even fewer have been based in Europe. The studies differ in methodology and estimates of prevalence from these studies range between 4 and 17 cases per 100 000.
The current study aims to increase knowledge about prevalence, clinical characteristics, and mortality in clinically diagnosed ATTR‐CM patients utilizing large datasets from four Nordic countries in Europe. To our knowledge, this is the first register‐based study of ATTR‐CM including several countries with inter‐country comparisons and aggregation of country‐level results as well as a comparison with a matched HF cohort.
Methods
Study design
A retrospective cohort study was conducted using national population‐based registers in four Nordic countries. To facilitate comparison and aggregation of results, the same methodology was used in all countries [with some minor country‐specific modifications when necessary (see below)]. The study period for patient identification was 1 January 2008 to 31 December 2018 in all countries and each patient was followed until death or end of study period. A period of up to 20 years before diagnosis was used to create covariates and apply exclusion criteria at the patient level.
Data sources
Patient‐level data were extracted from each country's national patient register, prescription drug register, and cause of death register. In each country, the registers were linked together using pseudonymized unique personal identifiers. The national patient registers provide information on diagnoses, procedures, and hospitalizations and outpatient specialist visits. The prescription drug registers contain data on all prescriptions filled at pharmacies. The cause of death registers provides the confirmed dates of death. National registers in all four countries have full coverage of the population and a high degree of completeness due to mandatory reporting.
Patient identification
Because no ATTR‐CM‐specific ICD‐10 code was available during the study period, patients were identified using a combination of diagnosis codes. The full study population included all adult (≥18 years of age) patients in each country with a diagnosis of amyloidosis (AM) (ICD‐10: E85.0, E85.1, E85.2, E85.4, E85.8, E85.9), cardiomyopathy (CM) (ICD‐10: I42.0, I42.1, I42.2, I42.5, I42.8, I42.9, I43.1, I43.8), or HF (ICD‐10: I50*) between 2008 and 2018 (Figure 1 ).
Figure 1.
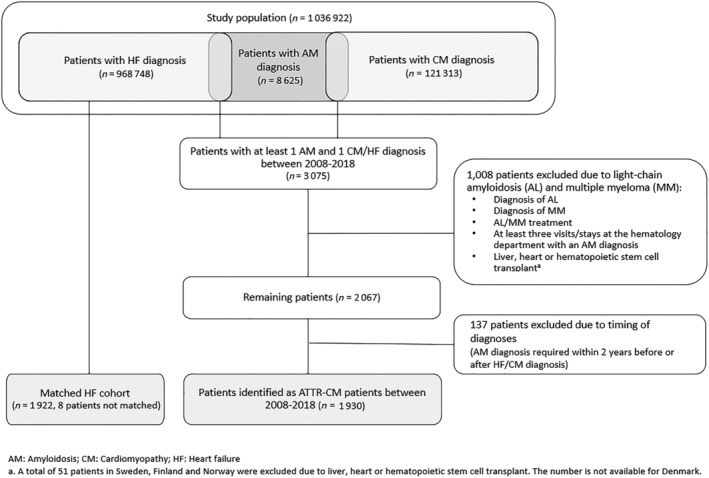
ATTR‐CM patient identification.
Transthyretin amyloid cardiomyopathy patients were identified from the study population by having a diagnosis of AM between 2008 and 2018 and at least one diagnosis of HF or CM within 2 years before or after the AM diagnosis. The date of the CM or HF diagnosis used for identification defined the index date (date of inclusion in the ATTR‐CM cohort). In turn, the index date was used as a proxy for the patient's first clinical manifestation of ATTR‐CM and is therefore referred to as the time of ATTR‐CM diagnosis throughout this manuscript. Patients with diagnosis of light‐chain (AL) amyloidosis or multiple myeloma (MM) at any time were excluded based on diagnosis codes, treatment, or visits to the haematology department, as well as patients with liver, heart, or haematopoietic stem cell transplant prior to AM diagnosis. The patient identification process was identical in the four countries and has been described elsewhere in detail. 10
The HF comparison cohort consisted of patients with a diagnosis of HF who were not included in the ATTR‐CM cohort (Figure 1 ). HF patients were matched to ATTR‐CM patients one‐to‐one with replacement, based on birthyear, sex, and the calendar year of diagnosis. The date of the first recorded HF diagnosis between 2008 and 2018 was used as the diagnosis date for patients in the HF comparison cohort.
Statistical analyses
All data management and statistical analyses were performed using R version 4.0 15 and Stata 16. 16 Confidence intervals (CI) are reported at the 95% level.
Pooling of individual‐level data across Nordic countries was not possible for legal reasons. Therefore, results from each country were aggregated as follows. A number of observations were summed up for all countries, and mean values were combined using the number of patients in each country as weight. Aggregated standard deviation was calculated using the formula:
where is the aggregated variance, is the variance in each country, and are patient numbers in country .
For Swedish, Finnish, and Norwegian country‐specific results, patient groups with less than five patients were censored to comply with the national data protection regulation. For Denmark, results for patient groups of less than 10 patients were censored for the same reason.
Characteristics
Sex and age of the patients were recorded at ATTR‐CM diagnosis. Co‐morbidities and pharmacy‐dispensed prescriptions of HF and cardiovascular medication were collected during a 3 year period and during a 1 year period from diagnosis, respectively. Note that some patients in Norway could have a less than 1 or 3 year lookback, as data collection started in 2008 in Norway. Co‐morbidities (primary and secondary diagnoses in inpatient and outpatient specialty care) were combined into the 31 category Elixhauser index, giving an indication of the patient's co‐morbidity burden. 17 Furthermore, diagnoses of potential early symptoms of ATTR‐CM (red flags), 18 listed in Table 1 , were gathered from start of data collection up to the time of diagnosis.
Table 1.
Clinical characteristics, prescribed medication and red flag diagnoses prior to diagnosis
| Denmark (N = 197) | Finland (N = 321) | Norway a (N = 418) | Sweden (N = 994) | Full ATTR‐CM cohort (N = 1930) | Full matched HF cohort (N = 1928) | |
|---|---|---|---|---|---|---|
| Females, n (%) | 40 (20.3) | 159 (49.5) | 102 (24.4) | 297 (29.9) | 598 (31.0) | 596 (30.9) |
| Age at diagnosis, mean b (SD) | 71.5 (12.0) | 73.6 (10.2) | 74.2 (12.7) | 72.7 (11.6) | 73.1 (3.4) | 73.1 (3.4) |
| Men | 71.3 (11.8) | 73.2 (9.7) | 74.1 (12.2) | 72.9 (11.2) | 73.0 (3.4) | 73.1 (3.4) |
| Women | 72.2 (13.1) | 74.0 (10.7) | 74.7 (14.0) | 72.2 (12.3) | 73.1 (3.5) | 73.2 (3.5) |
| Elixhauser co‐morbidity index, mean b (SD) | 3.23 (2.1) | 3.19 (1.9) | 5.50 (2.5) | 5.13 (2.4) | 4.69 (1.5) | 4.71 (1.5) |
| Men | 3.21 (2.0) | 3.12 (1.9) | 5.60 (2.6) | 4.91 (2.3) | 4.66 (1.5) | 4.75 (1.5) |
| Women | 3.30 (2.5) | 3.25 (2.0) | 5.20 (2.1) | 5.64 (2.7) | 4.77 (1.6) | 4.64 (1.5) |
| Prescriptions of heart and cardiovascular medication 1 year prior to diagnosis, n (%) | ||||||
| Beta‐blockers | 65 (33.0) | 208 (64.8) | 222 (53.1) | 584 (58.8) | 1079 (55.9) | 1119 (58.0) |
| ACE inhibitors | 46 (23.4) | 94 (29.3) | 119 (28.5) | 382 (38.4) | 641 (33.2) | 677 (35.1) |
| ARBs | 23 (11.7) | 90 (28.0) | 97 (23.2) | 292 (29.4) | 502 (26.0) | 453 (23.5) |
| Digoxin | 15 (7.6) | 30 (9.3) | 11 (2.6) | 74 (7.4) | 130 (6.7) | 132 (6.9) |
| Calcium channel blockers | 30 (15.2) | 84 (26.2) | 112 (26.8) | 226 (22.7) | 452 (23.4) | 530 (27.5) |
| Diuretics | 120 (60.9) | 201 (62.6) | 211 (50.5) | 608 (61.2) | 1140 (59.1) | 902 (46.8) |
| Antiplatelets | 44 (22.3) | 29 (9.0) | 160 (38.3) | 370 (37.2) | 603 (31.2) | 730 (37.9) |
| Lipid‐lowering agents | 45 (22.8) | 143 (44.5) | 186 (44.5) | 359 (36.1) | 733 (38.0) | 868 (45.0) |
| Anticoagulants | 49 (24.9) | 108 (33.6) | 137 (32.8) | 327 (32.9) | 621 (32.2) | 601 (31.2) |
| Red flags diagnoses prior to ATTR‐CM diagnosis, n (%) c | ||||||
| Carpal tunnel syndrome (unilateral and bilateral) | 24 (12.2) | 30 (9.3) | 48 (11.5) | 167 (16.8) | 269 (13.9) | 52 (2.7) |
| Spinal stenosis | 18 (9.1) | 41 (12.8) | 28 (6.7) | 86 (8.7) | 173 (9.0) | 61 (3.2) |
| Atrioventricular and left bundle‐branch block | 11 (5.6) | 25 (7.8) | 21 (5.0) | 84 (8.5) | 141 (7.3) | 83 (4.3) |
| Conductive and sensorineural hearing loss | ≤10 | 43 (13.4) | 77 (18.4) | 104 (10.5) | 224 (11.6) | 175 (9.1) |
| Atrial fibrillation and flutter | 53 (26.9) | 100 (31.2) | 147 (35.2) | 350 (35.2) | 650 (33.7) | 602 (31.2) |
| Other cardiac arrhythmias | ≤10 | 28 (8.7) | 44 (10.5) | 82 (8.2) | 154 (8.0) | 112 (5.8) |
| Other functional intestinal disorders | 12 (6.1) | 13 (4.0) | 22 (5.3) | 84 (8.5) | 131 (6.8) | 90 (4.7) |
| Other conduction disorders | 0 (0) | 11 (3.4) | ≤5 | 33 (3.3) | 44 (2.3) | 20 (1.0) |
| Supraventricular tachycardia | ≤10 | ≤5 | 7 (1.7) | 26 (2.6) | 33 (1.7) | 18 (0.9) |
| Sick sinus syndrome | ≤10 | 9 (2.8) | 9 (2.2) | 27 (2.7) | 45 (2.3) | 38 (2.0) |
| Non‐rheumatic aortic (valve) stenosis | 17 (8.6) | 15 (4.7) | 29 (6.9) | 39 (3.9) | 100 (5.2) | 106 (5.5) |
| Acute pericarditis | ≤10 | ≤5 | ≤5 | 8 (0.8) | 8 (0.4) | ≤5 |
| Primary pulmonary hypertension | ≤10 | 0 (0) | ≤5 | ≤5 | 0 (0) | ≤5 |
| Other secondary pulmonary hypertension | 0 (0) | 0 (0) | ≤5 | ≤5 | 0 (0) | ≤5 |
| Other specified cardiac arrhythmias | 0 (0) | ≤5 | ≤5 | 11 (1.1) | 11 (0.6) | 10 (0.5) |
| Irritable bowel syndrome | 0 (0) | 6 (1.9) | ≤5 | 18 (1.8) | 24 (1.2) | 24 (1.2) |
| Injury of muscle and tendon of long head of biceps | 0 (0) | 0 (0) | 0 (0) | ≤5 | 0 (0) | 0 (0) |
| Injury of muscle and tendon of other parts of biceps | 0 (0) | ≤5 | ≤5 | 0 (0) | 0 (0) | 0 (0) |
ACE, angiotensin‐converting enzyme; ARBs, angiotensin II receptor blockers; CCBs, calcium channel blockers; HF, heart failure; SD, standard deviation.
This includes Norwegian patients with ATTR‐CM diagnosis before 2011 who have less than 3 years of look‐back period as data collection in the Norwegian patient register started in 2008.
Means were calculated for each country‐specific population separately, aggregated means represent the mean values weighted by the number of patients in each country.
Ordered after absolute %‐point difference between ATTR‐CM and HF patients.
Prevalence
The annual prevalence of ATTR‐CM was calculated separately in each country by adding the ATTR‐CM patients alive at the beginning of a year and the new cases diagnosed during that year. This number was divided by the yearly country‐specific population (number of residents at 31 December). To obtain total aggregated prevalence in the Nordic countries, the number of ATTR‐CM patients (initial and new cases) and full national populations were summed by year.
Mortality
Kaplan–Meier estimates for all‐cause mortality were used to assess patients' survival after diagnosis. Aggregated results were based on summed country‐level data on number of events and censorings at each time point. The Mann–Whitney–Wilcoxon test was used to test for significance of differences in the aggregated survival curves.
Results
Patient identification
The full study population (patients with diagnosis of AM, CM, or HF between 2008 and 2018) in the four Nordic countries included a total of 968 748 patients with an HF diagnosis, 121 313 patients with a CM diagnosis and 8625 patients with an AM diagnosis (1 036 922 patients in total) (Figure 1 ). Of the 8625 patients with an AM diagnosis, 3075 patients were also diagnosed with CM/HF. A total of 1930 patients were included in the final ATTR‐CM cohort, after exclusion of patients with suspected AL or MM during the study period or with a heart, liver, or stem cell transplant before AM diagnosis (n = 1008) and exclusion of patients who did not fulfil the timing requirements for the diagnosis (n = 137). A total of 1928 patients were included in the HF comparison cohort as two patients failed to be matched to the ATTR‐CM cohort.
Characteristics
The mean age at diagnosis of ATTR‐CM patients in the Nordic countries was 73.1 years, ranging from 71.5 years (Denmark) to 74.2 years (Norway) between countries (Table 1 ). Aggregated across the countries, mean age for men was 73.0 years and 73.1 years for women. The largest within‐country difference in age at diagnosis between men and women was 0.9 years (Denmark).
The share of female patients was 20.3% in Denmark, 24.4% in Norway, and 29.9% in Sweden. In Finland, the distribution of men and women was found close to equal (49.5% women). Overall, in the Nordic countries, 31.0% of patients were female.
The mean Elixhauser co‐morbidity index was 4.69 in the combined Nordic countries, indicating that each ATTR‐CM patient had, on average, received diagnoses in five disease‐categories in the 3 years before AM diagnosis. Differences in the level of co‐morbidity index could be observed between countries; however, the co‐morbidity index for the ATTR‐CM and HF cohorts were similar in each country (Supporting Information, Table S1 ). In Denmark, the index was 3.23 for ATTR‐CM patients and 3.21 for the matched HF patient cohort; in Finland, it was 3.19 and 2.82; in Norway, it was 5.50 and 5.16, and in Sweden, it was 5.13 and 5.30, for ATTR‐CM and HF cohort, respectively. The mean co‐morbidity index in the combined Nordic countries was somewhat higher for women (4.77) compared with men (4.66).
More than half of ATTR‐CM patients in the Nordics were treated with at least one of the medications used in HF and cardiovascular disease (CVD), in the year before diagnosis (Table 1 ). The majority of patients in the ATTR‐CM cohort (59.1%) used diuretics in the year before diagnosis, compared with 46.8% of patients in the HF matched cohort. One per cent of patients (n = 27, all in Sweden) were treated with diflunisal; no other disease‐modifying treatments were used within the year before diagnosis. However, trends in medication use were reported before disease‐modifying or TTR targeted therapies were nationally approved and may have changed since.
The largest difference in share of red flag diagnoses between the aggregated ATTR‐CM cohort and the matched HF cohort was for carpal tunnel syndrome (13.9% vs. 2.7%, respectively) and spinal stenosis (9.0% vs. 3.2%, respectively). Similar trends were observed in all four countries (Table 1 and Supporting Information, Table S1 ).
Prevalence
In 2018, the mean prevalence over all countries was 3.3 per 100 000. Differences in prevalence between countries prevailed with 1.4 cases per 100 000 in Denmark, 1.8 in Finland, 3.7 in Norway, and 5.0 in Sweden (Figure 2 ). The prevalence increased in all four countries over the study period, most notably in Sweden and in Norway.
Figure 2.
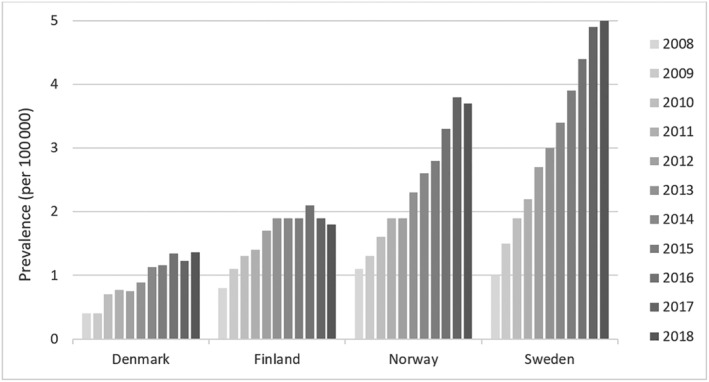
Prevalence by country and year.
Mortality
Median survival time after ATTR‐CM diagnosis varied in the four countries (Figure 3 ), but in each country, all‐cause mortality was significantly higher for ATTR‐CM patients compared with matched HF patients. In Denmark, median survival was 25 (CI: 21, 30) months and 70 (CI: 53, NA) months; in Finland, it was 18 (CI: 16, 22) months and 54 (CI: 47, 66) months; in Norway, it was 28 (CI: 25, 36) months and 59 (CI: 47, 70) months; and in Sweden, it was 37 (34, 44) months and 73 (64, 84) months for the ATTR‐CM and HF cohort, respectively. Accordingly, when aggregated over all patients in the four countries, survival for the ATTR‐CM cohort was significantly lower compared with the HF cohort (P < 0.0001) (Figure 4 ), with a median survival of 30 and 67 months for the ATTR‐CM cohort and HF cohort, respectively.
Figure 3.
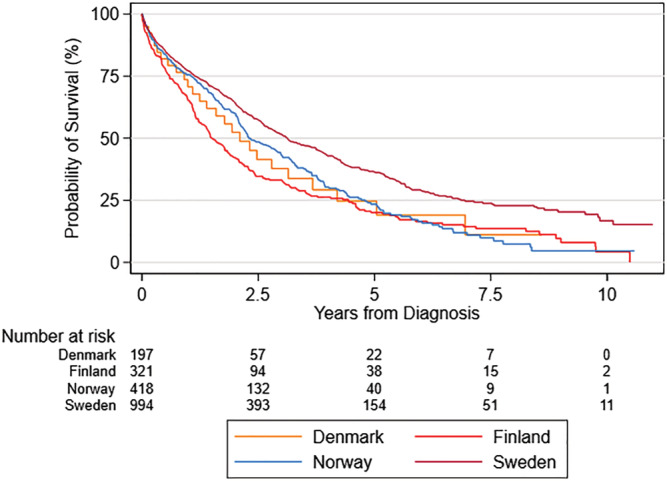
Mortality of ATTR‐CM patients in Denmark, Finland, Norway and Sweden.
Figure 4.

Aggregated mortality comparing ATTR‐CM cohort and matched HF cohort.
Overall, female ATTR‐CM patients had lower median survival compared with men, 22 months and 36 months, respectively. Aggregated survival was significantly lower for women than for men (P = 0.0001). For HF patients aggregated over the four countries, no significant difference in survival of men and women was observed (P = 0.4659) (Figure 5 ).
Figure 5.
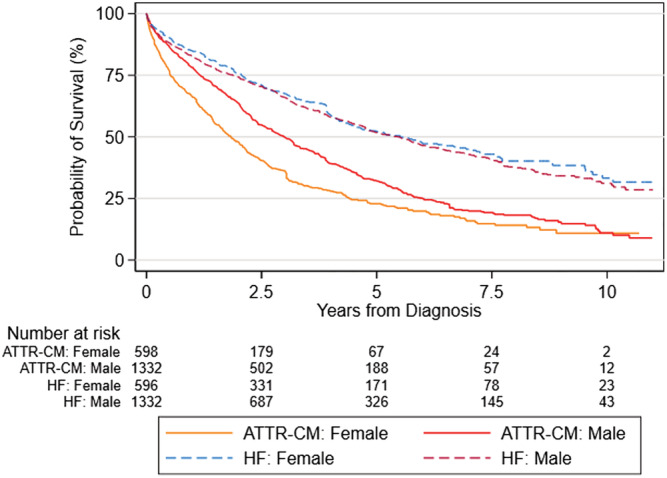
Aggregated mortality by gender for ATTR‐CM cohort and matched HF cohort.
Discussion
The aim of this retrospective study was to identify all clinically diagnosed ATTR‐CM patients in four Nordic countries (Denmark, Finland, Norway, and Sweden), describe their characteristics and to estimate the disease prevalence and mortality. A total of 1930 patients with ATTR‐CM were identified from national health registers.
The healthcare systems, standard of care, and available register data are very similar across the Nordic countries which makes these countries suitable for combined analyses. However, the countries differ somewhat in the type of inherent ATTR‐CM patients. Northern Sweden is known as endemic area for ATTRv amyloidosis with a Val30Met (p.Val50Met) mutation; the Swedish ATTRv amyloidosis population consists primarily of late onset cases which more frequently than early onset patients develop cardiac symptoms during the course of the disease 19 , 20 and are thereby most likely represented in the population identified in this study. The Leu111Met is the most common variant of ATTRv amyloidosis in Denmark and is commonly associated with ATTR‐CM. However, the total number of cases is low and likely too few cases are included to impact the study results. 9 Furthermore, if identified in time, patients with Leu111Met are treated with liver transplantation prior to development of HF and consequently excluded from this analysis. 21 No reports of ATTRv amyloidosis are available from Norway or Finland.
Prevalence
Sweden was found to have the highest prevalence of ATTR‐CM (5.0 per 100 000 in 2018). The relatively high prevalence of ATTRv amyloidosis likely impacts both the overall prevalence and the awareness among the healthcare professionals, resulting in more frequent diagnosis of ATTR‐CM. Instead, it is noteworthy that the countries with the lowest disease prevalence and highest mortality, Denmark and Finland, also have the lowest life expectancy of the Nordic countries which is why differences in prevalence may reflect higher burden of competing risks. 22 The lack of understanding of the aetiology of ATTRwt‐CM limits any conclusions regarding differences in pathophysiological mediators between the countries. In Sweden and Norway, prevalence increased steadily during the follow‐up from 2008 to 2018 likely reflecting increasing awareness over time. Only slight prevalence increase was observed in Denmark and Finland during the observed period, until 2018.
The Nordic‐wide prevalence estimate of 3.3 per 100 000 in 2018 is at the lower end compared with prevalence estimates ranging between 4 and 17 cases per 100 000 from Japan, the USA, and France. However, direct comparisons are difficult to make due to different methodologies used in studies, and a general lack of nationwide studies on prevalence of ATTR‐CM. The difficulty in obtaining comparable prevalence figures across regions and continents highlights the further need for prevalence studies.
Characteristics
Proportions of female ATTR‐CM patients in Denmark, Norway, and Sweden ranged between 20% and 30% and an equal share of female and male patients was found in Finland. These shares are higher compared with findings in meta‐analyses 8 , 11 and studies based on confirmed cases. 23 , 24 However, recent registry studies from France 12 and Japan 14 have reported higher estimates of female share, 33.1% and 53.7%, respectively. These recent results, based on unconfirmed cases, potentially suggest that women may be underdiagnosed. In Sweden, the higher share of women (30%) likely reflects the relatively higher proportion of ATTRv‐CM patients who exhibit a higher proportion of women compared with ATTRwt‐CM patients. 11 In Finland, we cannot exclude the possibility that the algorithm identified some patients with hereditary gelsolin (AGel) amyloidosis, a type of amyloidosis endemic to this country. However, only a small proportion of these patients develop heart failure or cardiomyopathy 25 which makes it an unlikely explanation of the finding. Age at diagnosis was 73 overall, which is consistent with the previous literature, 5 , 13 , 14 , 26 and differences between countries and men and women were rather small. The co‐morbidity index was similar in Norway (5.50) and Sweden (5.13) but differed from the co‐morbidity index found in Denmark (3.23) and Finland (3.19). However, very similar co‐morbidity index levels were observed for ATTR‐CM and HF patients within each country which suggests that the observed differences between countries may partly be explained by differences in clinical practice of registering multiple secondary diagnoses.
A higher degree of well‐established ATTR‐CM red flag diagnosis, like carpal tunnel syndrome and spinal stenosis, 18 was observed in the ATTR‐CM cohort compared with the HF cohort in all countries. This is an important indicator that patient identification in the present study was successful.
Mortality
Aggregated country‐level median survival time for patients with ATTR‐CM was 31 months which is in line with previous estimates 26 , 27 but less than half of the median survival of the matched HF cohort. Survival in the ATTR‐CM cohort was significantly shorter in all included countries. This underscores that ATTR‐CM represents an aggressive condition and clearly a distinct entity different from other forms of HF. It is important to consider that the survival estimates represent a time period (2008–2018) with very few effective treatments available, and that today the prognosis for (treated) patients likely has improved.
Estimates of mortality varied between the countries, with median survival times ranging from 40 months in Sweden to 18 months in Finland. The difference between Finland and Sweden could potentially be attributed to differences in age at diagnosis (SE: 72.7 years, FI: 73.6 years) but median survival in Norway, the country with the highest age at diagnosis (74.2), was 9 months higher than in Finland. This indicates that age at diagnosis likely is not a driving factor for median survival in this analysis. Instead, the higher median survival in Sweden may be due to higher awareness and earlier diagnosis of patients while the low survival prognosis in Finland may indicate that patients are diagnosed at later stages of the disease.
Overall, women in the ATTR‐CM cohort had a 42% lower median survival than men (22 months for women vs. 38 months for men) while age at diagnosis was similar (73.2 for women vs. 73.1 for men). No significant difference in male and female survival was observed in the aggregated HF cohort. The survival time was lower for women with ATTR‐CM in all countries, but the difference was statistically significant in Sweden and Norway. In Norway, women were on average 6 months older than men at diagnosis but had a lower co‐morbidity index. In Sweden, the co‐morbidity index was slightly higher for women, but age at diagnosis was lower. Thus, the registered demographic and clinical characteristics are likely not the sole driver of the lower median survival. Instead, ATTR‐CM may be detected at a later stage in women compared with men due to generally smaller heart size and wall‐thickness in women and as a result, potentially not meeting the threshold in diagnostic algorithm. 11 In addition, physicians observing heart failure with preserved ejection fraction (HFpEF) in female patients may also be less suspicious of ATTR‐CM because HFpEF is more prevalent in elderly women compared with elderly men. 28
Limitations
The data used in this study have certain limitations. We had to rely on diagnosis codes of amyloidosis, cardiomyopathy and HF for identification of ATTR‐CM patients, and thus, only patients diagnosed with a combination of these conditions could be included; no information on the clinical diagnostic process is available in the national registers. Because ATTR‐CM represents an underdiagnosed disease, true patient numbers are likely to be higher. Due to the lack of a specific ICD‐10 code for ATTR‐CM for the study period, patient identification was based on an algorithm and some non‐ATTR‐CM patients may have been included. Moreover, it was not possible to differentiate between ATTRwt‐CM, ATTRv‐CM and underlying mutations in the data, which complicates the interpretation of survival and prognosis. For the matched HF cohort, it was not possible to identify subtypes, such as HFpEF, which likely had improved the matching of the cohorts, due to the structure of the ICD‐10 codes. Even though health care systems and national registers are very similar in the four Nordic countries included in this study, it is important to acknowledge that ATTR‐CM patients were identified by an algorithm based on diagnosis codes and the differences in the usage of coding across countries impact the results. In addition, diagnosis of ATTR‐CM and thus prevalence may have increased even more after 2018, the last year observed in this study, given the launch of treatments and a general rise in attention to the disease in recent years.
Despite these limitations, this study provides important information on clinical characteristics, prevalence and mortality using nationwide data with near‐complete coverage in four countries. The identical study design allowed for comparison of patient groups in Denmark, Finland, Norway, and Sweden. The results are largely in line with previous findings and expectations which indicates a successful application of the patient identification algorithm.
Conclusions
In conclusion, this is the first study of ATTR‐CM including nationwide health registers from several countries and a relatively large population of almost 2000 ATTR‐CM patients. Results of this study confirm the severity of ATTR‐CM with median survival after diagnosis being significantly shorter than that of non‐ATTR matched heart failure patients. In particular, female ATTR‐CM patients seem to suffer from late diagnosis and demonstrate even higher mortality than male patients. Further studies are needed to verify the prevalence of ATTR‐CM in the Nordic countries.
Conflict of interest
Rosa Lauppe, Johan Liseth Hansen, and Anna Fornwall are employed by Quantify Research and funded by Pfizer to conduct this study; Quantify Research is a consultancy and works with a range of different pharmaceutical companies. Katarina Johansson, Mark H. Rozenbaum, Anne Mette Strand, and Merja Vakevainen are Pfizer employees and hold Pfizer stock and/or stock options. Johanna Kuusisto received support from Pfizer for her collaboration in this manuscript as well as grants or contracts from Sanofi‐Genzyme, Pfizer, and The Finnish Foundation for Cardiovascular Research. J.K. also received consulting fees and honoraria for lectures, presentations, speaker's bureaus, manuscript writing, or educational events from Sanofi‐Genzyme, Pfizer, Bayer, Takeda, Amgen, and Chiesi as well as payment for expert testimony from Sanofi‐Genzyme, Pfizer, Bayer, Takeda, and Amgen. J.K. has received support for participation on a Data Safety Monitoring Board or Advisory Board from Sanofi‐Genzyme, Pfizer, Bayer, Takeda, Amgen, and Chiesi and is supported by Amgen for Leadership or fiduciary role in other board, society, committee, or advocacy group. Einar Gude has received grants and honoraria for lectures from Pfizer. Finn Gustafsson has received support from Pfizer for work on the present manuscript and consulting fees from Pfizer, Alnylam, and Ionis. J Gustav Smith has no conflict of interests.
Funding
This work was supported by Pfizer AB.
Supporting information
Table S1. Clinical characteristics and prescribed medication prior to diagnosis in the HF cohort.
Acknowledgements
The authors thank M. D. Tuuli Mustonen for expert advice on gelsolin amyloidosis in Finland. Patrik Sandin is acknowledged for data analysis work.
Lauppe, R. , Liseth Hansen, J. , Fornwall, A. , Johansson, K. , Rozenbaum, M. H. , Strand, A. M. , Väkeväinen, M. , Kuusisto, J. , Gude, E. , Smith, J. G. , and Gustafsson, F. (2022) Prevalence, characteristics, and mortality of patients with transthyretin amyloid cardiomyopathy in the Nordic countries. ESC Heart Failure, 9: 2528–2537. 10.1002/ehf2.13961.
References
- 1. Rubin J, Maurer MS. Cardiac amyloidosis: Overlooked, underappreciated, and treatable. Annu Rev Med. 2020; 71: 203–219. [DOI] [PubMed] [Google Scholar]
- 2. Lane T, Fontana M, Martinez‐Naharro A, Quarta CC, Whelan CJ, Petrie A, Rowczenio DM, Gilbertson JA, Hutt DF, Rezk T. Natural history, quality of life, and outcome in cardiac transthyretin amyloidosis. Circulation. 2019; 140: 16–26. [DOI] [PubMed] [Google Scholar]
- 3. Grogan M, Scott CG, Kyle RA, Zeldenrust SR, Gertz MA, Lin G, Klarich KW, Miller WL, Maleszewski JJ, Dispenzieri A. Natural history of wild‐type transthyretin cardiac amyloidosis and risk stratification using a novel staging system. J Am Coll Cardiol. 2016; 68: 1014–1020. [DOI] [PubMed] [Google Scholar]
- 4. Ruberg FL, Grogan M, Hanna M, Kelly JW, Maurer MS. Transthyretin amyloid cardiomyopathy: JACC state‐of‐the‐art review. J Am Coll Cardiol. 2019; 73: 2872–2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pinney JH, Whelan CJ, Petrie A, Dungu J, Banypersad SM, Sattianayagam P, Wechalekar A, Gibbs SD, Venner CP, Wassef N. Senile systemic amyloidosis: Clinical features at presentation and outcome. J Am Heart Assoc. 2013; 2: e000098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Westin O, Butt JH, Gustafsson F, Schou M, Salomo M, Køber L, Maurer M, Fosbøl EL. Two decades of cardiac amyloidosis: A Danish Nationwide study. JACC: CardioOncology. 2021. 2021/08/17/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Norgren N, Andersson Escher S, Lundgren H‐E, Suhr OB, Olsson M. Genealogic studies of the Swedish hereditary transthyretin amyloidosis (ATTR V30M) population: differences in age at onset within the population. 2014.
- 8. Kroi F, Fischer N, Gezin A, Hashim M, Rozenbaum MH. Estimating the gender distribution of patients with wild‐type transthyretin amyloid cardiomyopathy: A systematic review and Meta‐analysis. Cardiol Ther. 2020; 10: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Damy T, Kristen AV, Suhr OB, Maurer MS, Planté‐Bordeneuve V, Yu CR, Ong ML, Coelho T, Rapezzi C. Transthyretin cardiac amyloidosis in continental Western Europe: An insight through the transthyretin amyloidosis outcomes survey (THAOS). Eur Heart J. 2019; 1: 391–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lauppe RE, Liseth Hansen J, Gerdesköld C, Rozenbaum MH, Strand AM, Vakevainen M, Kuusisto J, Gude E, Gustafsson F, Smith JG. Nationwide prevalence and characteristics of transthyretin amyloid cardiomyopathy in Sweden. Open Heart. 2021; 8: e001755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bruno M, Castaño A, Burton A, Grodin JL. Transthyretin amyloid cardiomyopathy in women: Frequency, characteristics, and diagnostic challenges. Heart Fail Rev. 2020. 1‐11.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Damy T, Bourel G, Slama M, Algalarrondo V, Lairez O, Pelcot F, Durand‐Zaleski I, Lilliu H, Bartoli M, Fievez S. PCV67 epidemiology of transthyretin amyloid cardiomyopathy (ATTR‐CM) in France: EPACT, a study based on the French Nationwide claims database Snds. Value Health. 2020; 23: S498–S499. [Google Scholar]
- 13. Gilstrap LG, Dominici F, Wang Y, El‐Sady MS, Singh A, Di Carli MF, Falk RH, Dorbala S. Epidemiology of cardiac amyloidosis‐associated heart failure hospitalizations among fee‐for‐service Medicare beneficiaries in the United States. Circ Heart Fail. 2019; 12: e005407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Winburn I, Ishii T, Sumikawa T, Togo K, Yasunaga H. Estimating the prevalence of transthyretin amyloid cardiomyopathy in a large in‐hospital database in Japan. Cardiol Ther. 2019; 8: 297–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. R Core Team . R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2021. [Google Scholar]
- 16. StataCorp . Stata Statistical Software: Release 16. College Station, TX: StataCorp LLC; 2021. [Google Scholar]
- 17. Garland A, Fransoo R, Olafson K, Ramsey C, Yogendran M, Château D. The Epidemiology and Outcomes of Critical Illness in Manitoba. 2012. 04/01.
- 18. Maurer MS, Bokhari S, Damy T, Dorbala S, Drachman BM, Fontana M, Grogan M, Kristen AV, Lousada I, Nativi‐Nicolau J. Expert consensus recommendations for the suspicion and diagnosis of transthyretin cardiac amyloidosis. Circ Heart Fail. 2019; 12: e006075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Holmgren G, Costa PM, Andersson C, Asplund K, Steen L, Beckman L, Nylander PO, Teixeira A, Saraiva MJ, Costa PP. Geographical distribution of TTR met30 carriers in northern Sweden: Discrepancy between carrier frequency and prevalence rate. J Med Genet. 1994; 31: 351–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Suhr OB, Lindqvist P, Olofsson BO, Waldenström A, Backman C. Myocardial hypertrophy and function are related to age at onset in familial amyloidotic polyneuropathy. Amyloid. 2006; 13: 154–159. [DOI] [PubMed] [Google Scholar]
- 21. Nelson LM, Penninga L, Villadsen GE, Mølgaard H, Eiskjær H, Hillingsø JG, Rasmussen A. Outcome in patients treated with isolated liver transplantation for familial transthyretin amyloidosis to prevent cardiomyopathy. Clin Transplant. 2015; 29: 1098–1104. [DOI] [PubMed] [Google Scholar]
- 22. Knudsen AK, Allebeck P, Tollånes MC, Skogen JC, Iburg KM, McGrath JJ, Juel K, Agardh EE, Ärnlöv J, Bjørge T, Carrero JJ, Cederroth CR, Eggen AE, El‐Khatib Z, Ellingsen CL, Fereshtehnejad S‐M, Gissler M, Hadkhale K, Havmoeller R, Johansson L, Juliusson PB, Kiadaliri AA, Kisa S, Kisa A, Lallukka T, Mekonnen T, Meretoja TJ, Meretoja A, Naghavi M, Neupane S, Nguyen TT, Petzold M, Plana‐Ripoll O, Shiri R, Sigurvinsdottir R, Skirbekk V, Skou ST, Sigfusdottir ID, Steiner TJ, Sulo G, Truelsen TC, Vasankari TJ, Weiderpass E, Vollset SE, Vos T, Øverland S. Life expectancy and disease burden in the Nordic countries: Results from the global burden of diseases, injuries, and risk factors study 2017. Lancet Public Health. 2019. 2019/12/01/; 4: e658–e669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rozenbaum MH, Ionescu I, Clausen M, Lopez M, Sultan MB, Attal S. Baseline characteristics of patients with transthyretin amyloid cardiomyopathy enrolled in a tafamidis expanded access programme. European Society of Cardiology (ESC) Congress 2021 – The Digital Experience2021. 42 [Google Scholar]
- 24. Lairez O, Reant P, Habib G, Jeanneteau J, Eicher JC, Jobbe Duval A, Lequeux B, Bauer F, Bartoli M, Noirot‐Cosson C, Rudant J, Kharoubi M, Damy T, editors. Demographic Characteristics of the 1902 Transthyretin Amyloid Cardiomyopathy Patients Treated By Tafamidis Through the French Early Access Program. European Society of Cardiology (ESC) Congress 2021 – The Digital Experience; 2021.
- 25. Schmidt E‐K, Mustonen T, Kiuru‐Enari S, Kivelä TT, Atula S. Finnish gelsolin amyloidosis causes significant disease burden but does not affect survival: FIN‐GAR phase II study. Orphanet J Rare Dis. 2020; 15: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Maurer MS, Elliott P, Comenzo R, Semigran M, Rapezzi C. Addressing common questions encountered in the diagnosis and Management of Cardiac Amyloidosis. Circulation. 2017; 135: 1357–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Elliott P, Drachman B, Gottlieb S, Hoffman J, Hummel S, Lenihan D, Ebede B, Gundapaneni B, Schwartz J, Sultan M. Interim analysis of data from a long‐term, extension trial of tafamidis meglumine in patients with transthyretin amyloid cardiomyopathy. Eur Heart J. 2019; 40: ehz748 0011. [Google Scholar]
- 28. Lam CSP, Donal E, Kraigher‐Krainer E, Vasan RS. Epidemiology and clinical course of heart failure with preserved ejection fraction. Eur J Heart Fail. 2011; 13: 18–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Clinical characteristics and prescribed medication prior to diagnosis in the HF cohort.


