FIGURE 9.
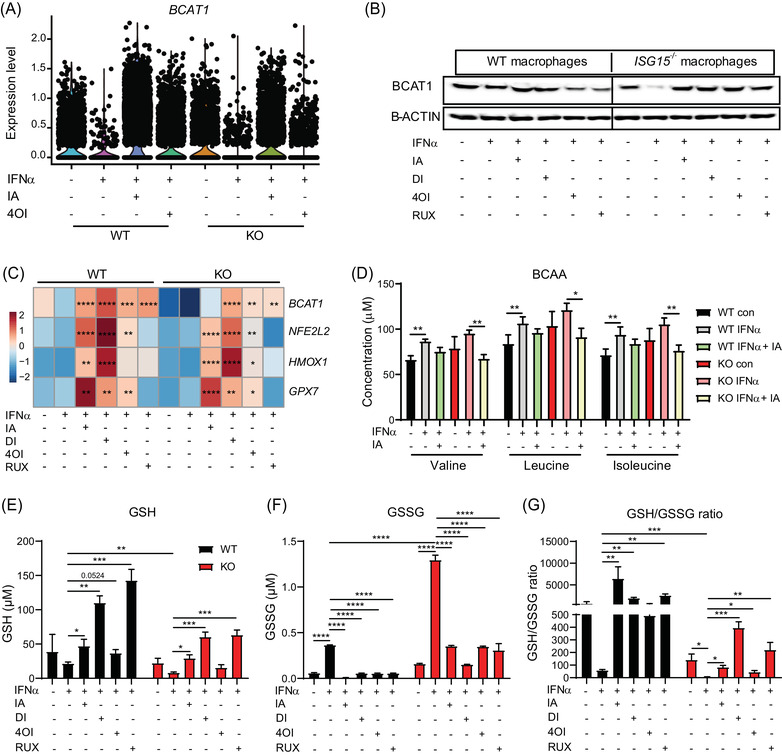
Itaconates and RUX rescue BCAT1 expression and redox balance in IFN‐α stimulated ISG15–/– cells, but only itaconates induce expression of anti‐oxidant genes NFE2L2, HMOX1, and GPX7. (A–E) WT and ISG15–/– iPSC‐derived macrophages were stimulated with IFN‐α (1000 IU/ml) for 8 h and treated with itaconate (IA, 10 mM), DI (0.5 mM), 4OI (25 µM), and RUX (1 µM) for 16 h. (A,B) Treatments rescue IFN‐α induced downregulation of BCAT1 mRNA (scRNAseq, each dot represents one cell) and BCAT1 protein (western blot of cell lysates). (C) Itaconates and RUX upregulate BCAT1 mRNA in IFN‐α stimulated WT and ISG15–/– cells, but only itaconates increase expression of anti‐oxidant genes NFE2L2, HMOX1, and GPX7 (RT‐qPCR). Statistical significance was determined with respect to the untreated, IFN‐α stimulated WT or ISG15–/– group. (D) WT and ISG15–/– dermal fibroblasts were stimulated with IFN‐α (1000 IU/ml) for 8 h and treated with itaconate (25 mM) for 16 h. Concentrations of BCAA valine, leucine, and isoleucine were measured by mass spectrometry. Itaconate treatment reduces levels of all three in IFN‐α treated ISG15–/– cells, indicating increased BCAT1 activity. (E–G) WT and ISG15–/– iPSC‐derived macrophages were stimulated with IFN‐α (1000 IU/ml) for 8 h and treated with itaconate (IA, 10 mM), DI (0.5 mM), 4OI (25 µM), and RUX (1 µM) for 16 h. GSH (reduced glutathione) and GSSG (oxidized glutathione) concentrations were measured with a fluorescence microplate reader (Ex/Em = 490/520 nm). Treatments increase GSH and decreased GSSG concentrations, resulting in improved redox balance (higher GSH/GSSG ratio). *, p < .05; **, p < .01; ***, p < .001; ****, p < .0001, one‐way ANOVA followed by Tukey's post hoc test
