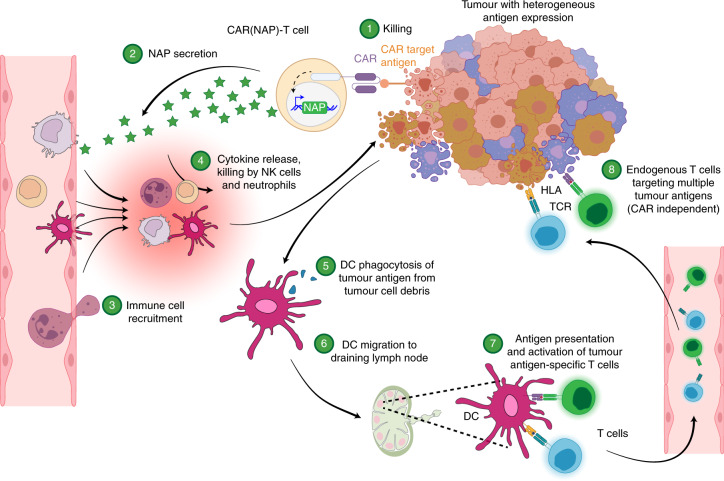
Fig. 5. Proposed mode of action of CAR(NAP) T-cell therapy.
CAR(NAP) T cells are CAR T cells armed with NAP, an immune activator derived from H. pylori. Within a solid tumour, heterogeneity in antigen expression makes it difficult to eradicate all tumour cells with conventional CAR T cells. CAR(NAP) T cells secrete NAP during killing of tumour cells (Steps 1 and 2). NAP works as a chemoattractant, recruiting innate immune cells, such as neutrophils and NK cells, and activates them to directly kill tumour cells independent of whether they express the CAR target or not (Steps 3 and 4). NAP also recruits monocytes and DCs, and activates them to secrete pro-inflammatory cytokines that directly counteract the hostile tumour microenvironment and strengthen the function of CAR(NAP) T cells. Immature DCs can phagocytose cell debris from dying tumour cells (Step 5). During this process, the DCs mature and migrate to a draining lymph node (Step 6) where they degrade the tumour cell debris and present peptides, including tumour-associated antigens, on the human leukocyte antigen (HLA) molecules to naive T cells. T cells with matching T-cell receptors (TCRs) recognize tumour-associated antigen epitopes and become activated (Step 7). The newly activated T cells recirculate back to the tumour site and kill the tumour cells through TCR-mediated recognition, which is independent of the antigen targeted by the CAR (Step 8). Therefore, this technology can counteract heterogeneity in antigen expression within solid tumours.