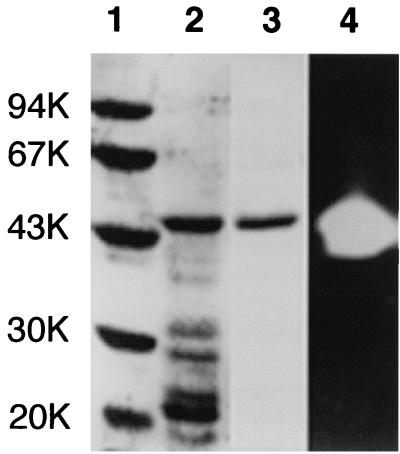
FIG. 2.
Comparison of the purity of T. kodakaraensis subtilisin by SDS-PAGE. Samples were subjected to electrophoresis on a 12% polyacrylamide gel in the presence of SDS. After electrophoresis, the gel was stained with Coomassie brilliant blue (lanes 1 to 3) or stained for protease activity (lane 4). Lane 1, low-molecular-weight, marker kit (Pharmacia Biotech) containing phosphorylase b, bovine serum albumin, ovalbumin, carbonic anhydrase, trypsin inhibitor, and α-lactalbumin; lanes 2 and 4, refolding sample of insoluble fractions obtained from E. coli BL21-codonPlus(DE3) harboring plasmid pET25b-Tk- subtilisin upon lysis by sonication; lane 3, purified T. kodakaraensis subtilisin. The molecular weight (in thousands [K]) of each standard protein is indicated to the left of the gel.