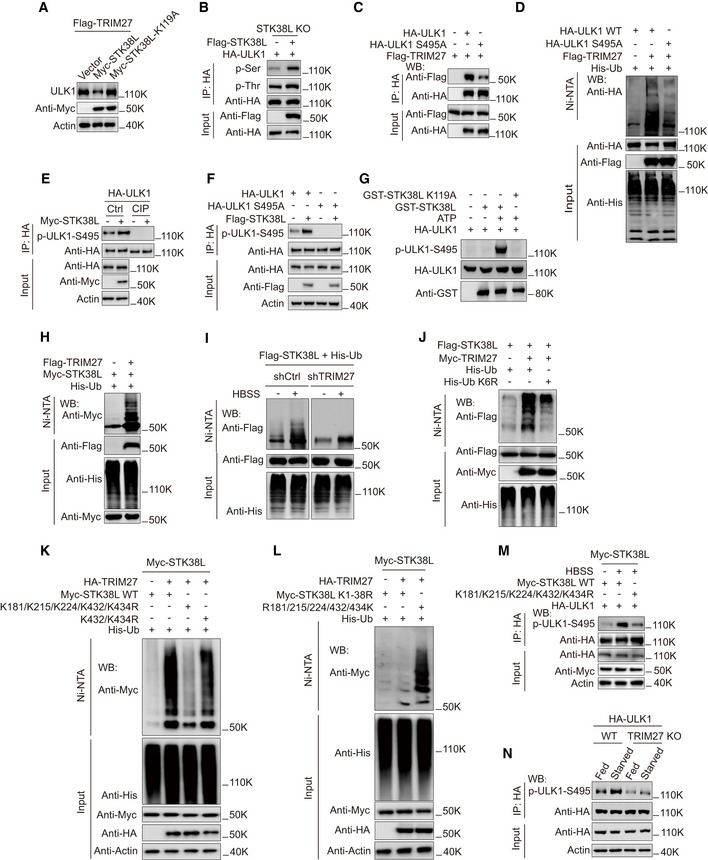
Figure 5. TRIM27‐mediated ubiquitination activates STK38L to phosphorylate ULK1.
-
AWestern blot analysis of ULK1 levels in HeLa cells stably expressing Flag‐TRIM27, the cells were transfected with either Myc‐vector, Myc‐STK38L, or Myc‐STK38L‐K119A. Then the cells were treated with starvation before lysis.
-
BPhosphorylation of HA‐ULK1 in HeLa STK38L knockout cells with or without expression of Flag‐STK38L. HA‐ULK1 was immunoprecipitated and then analyzed by Western blot using anti‐phosphoserine or anti‐phosphothreonine, and the cell lysates were analyzed using anti‐HA and anti‐Myc.
-
CCo‐immunoprecipitation of Flag‐TRIM27 with HA‐ULK1 or HA‐ULK1‐S495A in HEK293T cells. HA‐ULK1 or HA‐ULK1 S495A was immunoprecipitated using anti‐HA, the immunoprecipitates and lysates were analyzed with anti‐HA or anti‐Flag.
-
DUbiquitination analysis of HA‐ULK1 WT or HA‐ULK1 S495A in HEK293T cells co‐expressing Flag‐TRIM27 and His‐Ub.
-
EPhosphorylation of HA‐ULK1 in HEK293T cells co‐expressing Myc‐STK38L with or without CIP treatment. HA‐ULK1 was immunoprecipitated with anti‐HA and analyzed by Western blot using a specific antibody against p‐ULK1‐S495 (Ser495).
-
FPhosphorylation of HA‐ULK1 or HA‐ULK1 S495A in HEK293T cells co‐expressing Myc‐STK38L. HA‐ULK1 or HA‐ULK1 S495A was immunoprecipitated with anti‐HA and analyzed by Western blot using a specific antibody against p‐ULK1‐S495 (Ser495).
-
GIn vitro kinase assay using GST‐STK38L, or GST‐STK38L K119A (a kinase‐inactive mutant) and HA‐ULK1 as substrate. GST‐STK38L or GST‐STK38L K119A was pulled down using glutathione Sepharose beads. HA‐ULK1 was immunoprecipitated from HEK293T using anti‐HA and treated with lambda phosphatase, the reaction product was analyzed by Western blot using p‐ULK1‐S495 (Ser495).
-
HUbiquitination analysis of Myc‐STK38L in HEK293T cells co‐expressing Flag‐TRIM27 and His‐Ub.
-
IUbiquitination analysis of Flag‐STK38L in HEK293T cells co‐expressing His‐Ub with or without TRIM27 knockdown, cells were cultured in fed or starved conditions as indicated.
-
JUbiquitination analysis of Flag‐STK38L in HEK293T cells co‐expressing Myc‐TRIM27 with either His‐Ub (wild‐type) or His‐Ub K6R.
-
KUbiquitination analysis of Myc‐STK38L WT, Myc‐STK38L‐K432R+K434R or Myc‐STK38L‐K181R+K215R+K224R+K432R+K434R (Myc‐STK38L‐5KR) mutant in HEK293T cells co‐expressing HA‐TRIM27 and His‐Ub.
-
LUbiquitination analysis of Myc‐STK38L K1‐38R mutant (All lysines were mutated to arginines), or Myc‐STK38L‐R181/215/224/432/434K (All lysines were mutated to arginines except the indicated five lysine residues) mutant in HEK293T cells co‐expressing HA‐TRIM27 and His‐Ub.
-
MPhosphorylation of HA‐ULK1 S495(Ser495) in HEK293T cells co‐expressing Myc‐STK38L WT, or Myc‐STK38L‐5KR mutant in K. The cells were cultured in fed or starved conditions as indicated and HA‐ULK1 was immunoprecipitated using anti‐HA, the immunoprecipitates and lysates were analyzed with a specific antibody against p‐ULK1‐S495 (Ser495), anti‐Myc, anti‐HA, or anti‐actin.
-
NPhosphorylation of HA‐ULK1 in HeLa cells with or without TRIM27‐knockout, cells were cultured in fed or starved conditions as indicated, HA‐ULK1 was immunoprecipitated using anti‐HA, the immunoprecipitates and lysates were analyzed with a specific antibody against p‐ULK1‐S495 (Ser495), anti‐HA, or anti‐actin.
Source data are available online for this figure.
