Highlights
-
•
The clinical incidence of spinal metastases is 15.67%, two thirds are metastases from breast-, prostate- or lung cancer.
-
•
9.6% of patients with spinal metastases develop metastatic epidural spinal cord compression.
-
•
1 out of 8 (12.6%) of patients with spinal metastases suffer of pathologic vertebral compression fractures.
Abbreviations: CA, carcinoma; CI, confidence interval; HCC, hepatocellular carcinoma; LOL, length of life; MESCC, metastastic epidural spinal cord compression; MRI, magnetic resonance imaging; OR, odds ratio; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; pVCF, pathologic vertebral compression fractures; QOL, quality of life; RCT, randomized controlled trial; rMESCC, subclinical radiographic MESCC; SINS, spinal instability neoplastic score; SM, spinal metastases; SR, systematic review; SRE, skeletal related event; ST, solid tumor; STROBE, Strengthening the reporting of observational studies in epidemiology; WHO, World Health Organization
Keywords: Spinal metastases, Metastatic epidural spinal cord compression, Pathologic vertebral compression fracture, Oncology, Epidemiology
Abstract
Introduction
Spinal metastases (SM) are a frequent complication of cancer and may lead to pathologic vertebral compression fractures (pVCF) and/or metastatic epidural spinal cord compression (MESCC). Based on autopsy studies, it is estimated that about one third of all cancer patients will develop SM. These data may not provide a correct estimation of the incidence in clinical practice.
Objective
This systematic review (SR) aims to provide a more accurate estimation of the incidence of SM, MESCC and pVCF in a clinical setting.
Methods
We performed a SR of papers regarding epidemiology of SM, pVCF, and MESCC in patients with solid tumors conform PRISMA guidelines. A search was conducted in the PubMed and Web of Science database using the terms epidemiology, prevalence, incidence, global burden of disease, cost of disease, spinal metastas*, metastatic epidural spinal cord compression, pathologic fracture, vertebral compression fracture, vertebral metastas* and spinal neoplasms. Papers published between 1975 and august 2021 were included. Quality was evaluated by the STROBE criteria.
Results
While 56 studies were included, none of them reports the actual definition used for MESCC and pVCF, inevitably introducing heterogenity. The overall cumulative incidence of SM and MESCC is 15.67% and 2.84% respectively in patients with a solid tumor. We calculated a mean cumulative incidence in patients with SM of 9.56% (95% CI 5.70%-13.42%) for MESCC and 12.63% (95% CI 7.00%-18.25%) for pVCF. Studies show an important delay between onset of symptoms and diagnosis.
Conclusions
While the overall cumulative incidence for clinically diagnosed SM in patients with a solid tumor is 15.67%, autopsy studies reveal that SM are present in 30% by the time they die, suggesting underdiagnosing of SM. Approximately 1 out of 10 patients with SM will develop MESCC and another 12.6% will develop a pVCF. Understanding these epidemiologic data, should increase awareness for first symptoms, allowing early diagnosis and subsequent treatment, thus improving overall outcome.
1. Introduction
The world health organization (WHO) estimates an exponential increase of the incidence of cancer with 29.4 million new cases in 2040 [1]. In addition, life expectancy and the quality of life (QOL) of these patients are improving [2]. This results in a growing population living with solid primary tumors, who are at risk to develop spinal metastases (SM), pathologic vertebral compression fractures (pVCF) and metastatic epidural spinal cord compression (MESCC).
Spinal metastases (SM) are a frequent cause of oncologic pain. Moreover, they may lead to pathologic vertebral compression fractures (pVCF) and/or metastatic epidural spinal cord compression (MESCC).
In recent years QOL has emerged as an important outcome parameter next to survival. QOL and length of life (LOL) are intertwined outcome parameters, as QOL has been demonstrated a prognostic factor for survival [3], [4], [5].
MESCC may impair gait to the point that autonomic mobility is lost, severely affecting QOL. While urgent treatment improves the odds of functional recovery, neurological recovery is compromised, once function has been lost [6], [7]. After publication of the frequently cited Patchell study (2005), standard care for MESCC consists of decompressive surgery followed by conventional radiotherapy [8]. In subsequent years randomized controlled trials (RCTs) regarding this subject have been scarce. As treatment without delay and above all before mobility is lost, leads to a better functional outcome [6], [7], [8], early recognition and diagnosis are of primordial importance.
SM may lead to spinal instability. In 2010 Fisher et al. published the Spinal Instability Neoplastic Score (SINS), a validated comprehensive classification system to guide surgical decision making. Patients with spinal instability are at risk for (movement-related) pain and pVCF, potentially leading to compression of neurological structures [9].
Interestingly, literature regarding the incidence of SM mostly refers to relatively old autopsy studies [10], [11]. Given the enormous evolution in cancer therapies over the past decades, it might be of interest to critically review all available data on the incidence of SM and MESCC. In contrast to the registration of primary tumors, formal registration of SM, pVCF, and MESCC is lacking in most registries. A better knowledge of the incidence of MESCC should improve awareness for early clinical symptoms and radiological signs preceding significant loss of function. Early diagnosis may help to preserve mobility, independency, and overall QOL.
This systematic review (SR) aims to provide an evidence based estimation of the cliinical incidence of SM, MESCC, and pVCF in patients with solid primary tumors.
2. Methods
This literature review was conducted in line with the guidelines of Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [12].
A search between January 1st 1975 and august 31st 2021 was conducted in PubMed and Web-of-Science using the following search-terms: epidemiology, prevalence, incidence, global burden of disease, spinal metastas*, metastatic epidural spinal cord compression, pathologic fracture, vertebral compression fracture, vertebral metastas*, spinal neoplasms. Reference lists of review studies and papers included in the review were screened for any other papers that included these key-terms.
Studies had to match the following inclusion criteria: (1) published in English with full text available; (2) original study; (3) containing data on the epidemiology of SM, pVCF and/or MESCC; (4) focusing on the general population or the population with a solid primary tumor, not merely a specific subgroup (e.g. surgically treated patients, due to an inherent risk of bias); (5) adults.
Retrieved papers were selected by screening title (first step), abstract (second step), and content (third step) independently by two researchers (RVdB and EC).
Extracted data included source population, study period, source population with solid tumor, population with SM, MESCC and pVCF.
We based our evaluation of the methodological quality on five elements in the STROBE checklist which were most relevant to the quality of the reported incidence and mortality rates: study design, setting, participants, data sources/measurements, and study size [13].
Data were extracted and cumulative incidences in patients with solid tumor and in patients with SM were calculated by dividing the number of patients with respectively SM, MESCC, or pVCF by the total number of study subjects or the number of patients with SM. Descriptive statistics by GraphPad (https://www.graphpad.com) were used to calculate means and 95% confidence intervals (CI).
3. Results
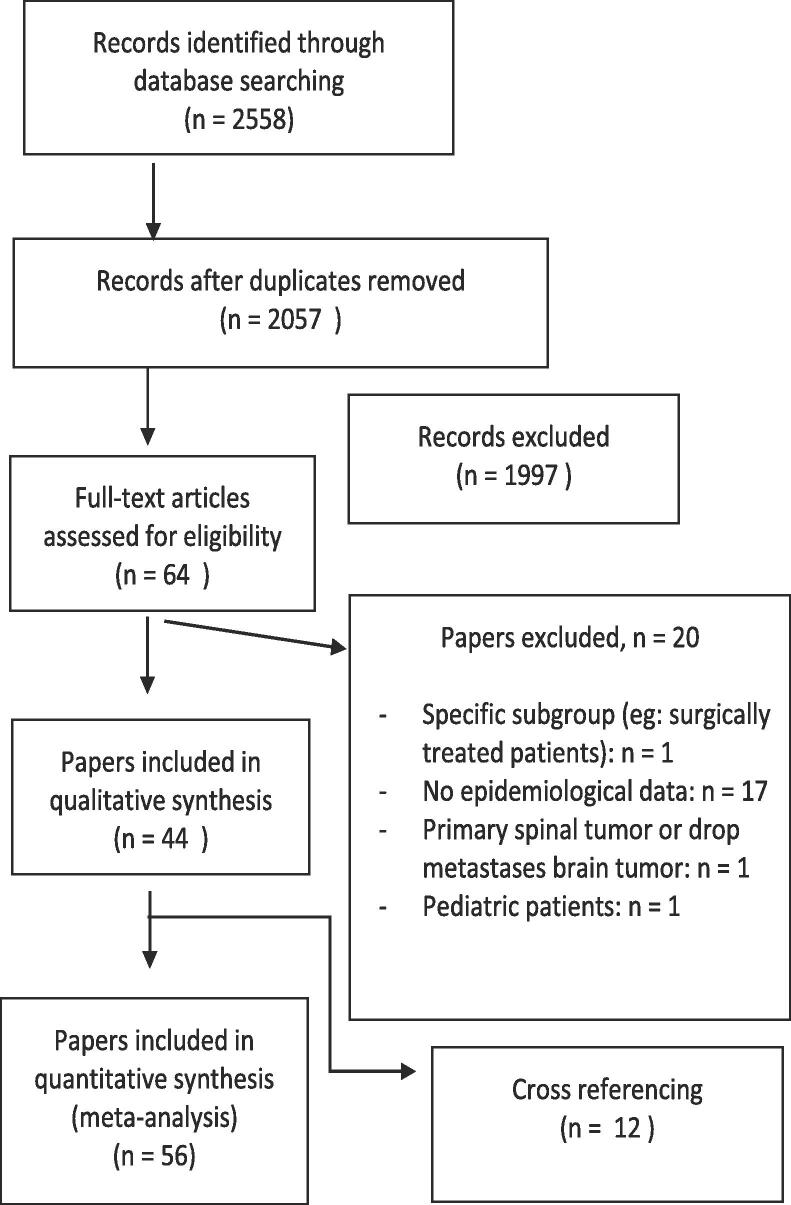
PubMed and Web of Science search identified 2057 papers, after removing duplicates. Following screening we retained 44 papers, added another 12 papers after cross referencing. We included 56 papers for final analysis (Fig. 1, Table 1, Table 2).
Fig. 1.
PRISMA flow diagram of the literature search and selection of papers.
Table 1.
SM: spinal metastases, MESCC: metastatic epidural spinal cord compression, pVCF: pathologic vertebral compression fracture. †: subgroup of this study; ‡: cumulative incidence of MESCC in the 5 years preceding death from cancer. Cumulative incidence = No. of patients with SM/MESCC/pVCF divided by the total number of study subjects; §: data not shown in Fig. 2 due to axis limits. ¶: Risk of bias: 1: properly powerd and conducted RCT, 2: well-designed controlled trial without randomization; prospective comparative cohort trial, 3: case-control studies; retrospective cohort study, 4: case series with or without intervention; cross-sectional study, 5: opinion of respected authorities, case reports.
| Author | Study population | Patients with solid tumour | Patients with spinal metastasis | Patients with MESCC | Patients with pVCF | SM per 1000 patients with solid tumor | MESCC per 1000 patients with solid tumor | MESCC per 1000 patients with SM | pVCF per 1000 patients with solid tumor | pVCF per 1000 patients with SM | STROBE quality criteria | Risk of bias |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Autopsy studies | ||||||||||||
| Fornasier 1975 [13] | 643 autopsies in an oncologic center | 374 | 140 | / | / | 374,3 | / | / | / | / | Complete | 3 |
| Wong 1988 [14] | 832 autopsies of patients with a terminal diagnosis of malignant neoplasm | 612 | 248 | / | / | 405,2 | / | / | / | / | Complete | 3 |
| Ortiz Gómes 1995 [22] | 842 autopsies in patients dying from malignant neoplasm | 734 | 225 | / | / | 306,5 | / | / | / | / | Complete | 4 |
| All tumors | ||||||||||||
| Bach 1990 [23] | Retrospective population based, Denmark, 1979-1983 | / | / | 398 | / | / | 4.4% to 6% | / | / | / | 4/5 | 3 |
| Loblaw 2003 [24] | Retrospective population, Ontario, Canada, 1990-1995 | 121.435 | / | 3.458 | / | / | 25,4 | / | / | / | 4/5 | 3 |
| Schulman 2006 [15] | Retrospective database, US, 2000-2004 | 396.200 | 18.042 | / | / | 45,5 | / | / | / | / | Complete | 4 |
| Zaikova 2009 [25] | Retrospective population based, South-East Norway, 2007-2008 | 17.757 | 1.002 | 313 | / | 56,4 annual incidence per 100.000 inhabitants: 26,0 | 17,6 annual incidence per 100.000 inhabitants: 8,1 | 312,3 § | / | / | 4/5 | 3 |
| Mak 2011 [17] | Retrospective database, US, 1998-2006, (20% of the US population) | / | / | 15.367 | / | / | 34,5 | / | / | / | 4/5 | 4 |
| Oster 2013 [19] | Retrospective database, US, 1995-2009 | 35.692 | 1.819 | / | / | 50,6 | / | / | / | / | Complete | 4 |
| Sohn 2015 [26] | Retrospective population based cohort, Korea, 2009-2012 | / | 13.288 | / | / | 6.04-6.83 per 100.000 persons per year | / | / | / | / | 4/5 | 3 |
| Phanphaisarn 2016 [27] | Retrospective cohort, Thailand, 2006-2015 | 29.447 | 2.263 | 165 | 120 | 76,8 | 5,6 | 72,9 | 4,1 | 53,0 | 4/5 | 3 |
| Wild 2016 [28] | Retrospective cohort, Germany | / | 848 | / | 94 | / | / | / | / | 110,8 | 3/ 5 | 4 |
| Svensson 2017 [29] | Retrospective population based cohort, Denmark, 1994-2010 | 176.722 | 17.251 | / | / | 97,6 | / | / | / | / | Complete | 3 |
| Hernandez 2018 [21] | Retrospective database, US, 2004-2013 | 382.733 | 26.250 | / | / | 68,5 | / | / | / | / | Complete | 4 |
| Campillo-Recio 2019 [30] | Retrospective cohort; paliative care unit, Madrid, Spain, 2008-2016 | 1.736 | / | 28 | / | / | 16,1 | / | / | / | 4/5 | 3 |
| Hong 2020 [16] | Retrospective database, Korea, 2002-2013 | 21.562 | 1.849 | 63 | 201 | 85,8 | 2,9 | 34,1 | 9,3 | 108,7 | 4/5 | 4 |
| Choi 2020 [18] | Retrospective population based, South Korea, 2008-2017 | / | 38.007 | / | / | 6.68 cases per 100.000 population per year | / | / | / | / | Complete | 4 |
| Price 2021 [20] | Retrospective database, US, 2012-2014 | 51.800 | 18.752 | 38.591 | / | / | 362,0 § | / | 745,0 § | 4/5 | 4 | |
| Breast carcinoma | ||||||||||||
| Domcheck 2000[31] | Retrospective cohort women diagnosed with metastatic breast carcinoma, US, 1981-1991 | 718 | 420 | 61 | 56 | 585,0 | 85,0 § | 145,2 | 78,0 | 133,3 | 4/5 | 3 |
| Loblaw 2003† [24] | Retrospective population, Ontario, Canada, 1990-1995 | 35.197 | / | 689 | / | / | 19,6CI: 5.52%‡ | / | / | / | 4/5 | 3 |
| Oka 2006 [32] | Retrospective cohort, Tokio, 1990-1996 | 695 | 121 | 17 | / | 174,1 | 24,5 | 140,5 | / | / | 4/5 | 3 |
| Zaikova 2009† [25] | Retrospective population based, South-East Norway, 2007-2008 | 1.597 | / | / | / | 201 | 28 | / | / | / | 4/5 | 3 |
| Jensen 2011 [33]Yong 2011 [34] | Retrospective cohort, Denmark, 1999-2007 | 35.912 | 1.450 | 143 | 133 | 40,4 | 4,0 | 98,6 | 3,7 | 91,7 | 4/5 | 3 |
| Mak 2011 † [17] | Retrospective database, US, 1998-2006, (20% of the US population) | / | / | / | / | / | 16,8 | / | / | / | 4/5 | 4 |
| Oster 2013 † [19] | Retrospective database, US, 1995-2009 | 11.738 | 621 | / | / | 52.9 | / | / | / | / | Complete | 4 |
| Phanphaisarn 2016 † [27] | Retrospective cohort, Thailand, 2006-2015 | 4.050 | 335 | 26 | 22 | 82,7 | 6,4 | 77,6 | 5,4 | 65,7 | 4/5 | 3 |
| Svensson 2017 † [29] | Retrospective population based cohort, Denmark, 1994-2010 | 69.009 | 3.789 | / | 54,9 | / | / | / | / | Complete | 3 | |
| Hernandez 2018 a[21] | Retrospective database, US, 2004-2013 | 137.720 | / | / | / | 81 | / | / | / | / | Complete | 4 |
| Baek 2019 † [35] | Retrospective cohort, South-Korea, 2004-2015 | / | 23.811 | 341 | 2.260 | / | / | 14,3 | / | 94.9 | 4/5 | 4 |
| Hong 2020 † [16] | Retrospective database, Korea, 2002-2013 | 2.221 | 417 | 8 | 50 | 187,8 | 3,6 | 19,2 | 22,5 | 119,9 | 4/5 | 4 |
| Prostate carcinoma | ||||||||||||
| Kuban 1986 [36] | Retrospective cohort, Virginia US, 1975-1983 | 611 | / | 41 | / | / | 67,1 | / | / | / | 4/5 | 4 |
| Berruti 2000 [37] | Prospective cohort, Italy, 1990-1998 | / | 112 | 7 | 10 | / | / | 62,5 | / | 89,3 | 4/5 | 3 |
| Loblaw 2003 † [24] | Retrospective population, Ontario, Canada, 1990-1995 | 32.497 | / | 638 | / | 19,6Cumulative incidence° 7.24% | / | 4/5 | 3 | |||
| Zaikova 2009a[25] | Retrospective population based, South-East Norway, 2007-2008 | 2.458 | / | / | / | 297 | 17 | / | / | / | 4/5 | 3 |
| Norgaard 2010 [38] | Retrospective population based cohort 1999-2007 | 23.087 | 3.147 | 447 | 169 | 136,3 | 19,4 | 142,0 | 7,3 | 53,7 | 4/5 | 3 |
| Venkitaraman 2010 [39] | Retrospective cohort 2001-2005 | 570 | 130 | 57 | / | 228,1 | 100,0 c | 438,5§ | / | / | 4/5 | 3 |
| Mak 2011† [ 17] | Retrospective database, US, 1998-2006, (20% of the US population) | / | / | / | / | / | 54,9 | / | / | / | 4/5 | 4 |
| Oster 2013† [19] | Retrospective database, US, 1995-2009 | 14.866 | 721 | / | / | 48,5 | / | / | / | / | Complete | 4 |
| Onukwugha 2014 [40] | Retrospective database, US, 2000-2007 | 7.062 | / | 524 | 306 | / | 74,2 | / | 43,3 | / | 4/5 | 3 |
| Perrault 2015 [41] | Retrospective population-based cohort, Canada, 2001 | 2297 | 626 | 18 | 60 | 272,5 | 7,8 | 28,8 | 26,1 | 95,8 | 4/5 | 3 |
| Phanphaisarn 2016 † [27] | Retrospective cohort, Thailand, 2006-2015 | 715 | 154 | 12 | 8 | 215,4 | 16,8 | 77,9 | 11,2 | 51,9 | 4/5 | 3 |
| Svensson 2017 † [29] | Retrospective population based cohort, Denmark, 1994-2010 | 42.857 | 5.941 | / | / | 138,6 | / | / | Complete | 3 | ||
| Hernandez 2018 † [21 | Retrospective database, US, 2004-2013 | 22.801 | / | 292 | / | / | Complete | 4 | ||||
| Kawai 2019 [42] | Retrospective cohort study, US, 2000-2011 | 2.234 | / | 37 | 266 | / | 16,6 | / | 119,1 | / | 4/5 | 3 |
| Baek 2019 † [35] | Retrospective cohort, South-Korea, 2004-2015 | / | 19.170 | 467 | 2032 | / | / | 24,0 | / | 106.0 | 4 /5 | 4 |
| Hong 2020 † [16] | Retrospective database, Korea, 2002-2013 | 1.109 | 194 | 6 | 35 | 174,9 | 5,4 | 30,9 | 31.6 | 180,4 | 4/5 | 4 |
| Lung carcinoma | ||||||||||||
| Loblaw 2003† [24] | Retrospective population, Ontario, Canada, 1990-1995 | SC: 5.654NSCLC: 32.027 | / | 791 | / | / | 25,6 - 33,6 | / | / | / | 4/5 | 3 |
| Zaikova 2009a[25] | Retrospective population based, South-East Norway, 2007-2008 | 1.447 | / | / | / | 554c | 97 § | / | / | / | 4/5 | 3 |
| Mak 2011† [17] | Retrospective database, US, 1998-2006, (20% of the US population) | / | / | / | / | / | 17,4 | / | / | / | 4/5 | 4 |
| Decroisette 2011 [43] | Prospective cohort, France, 2006-2007 | / | 554 | 21 | 38 | / | / | 37,9 | / | 68,6 | 4/5 | 3 |
| Oster 2013 a[19] | Retrospective database, US, 1995-2009 | 9.088 | 477 | / | / | 52,5 | / | / | / | / | Complete | 4 |
| Cetin 2014 [44] | Retrospective population based cohort, Denmark, 1999-2010 | 29.720 | 2.032 | 240 | 92 | 68,4 | 8,1 | 118,1 | 3,1 | 45,3 | 4/5 | 3 |
| Katakami 2014 [45] | Prospective cohort, Japan, 2007-2009 | 274 | 78 | 3 | 14 | 284,7 | 10,9 | 38,5 | 51,1 | 179,5 | 4/5 | 3 |
| Dalgaard 2015 [46] | Retrospective cohort, Denmark, 2003-2009 | 28443 | 1668 | / | / | 58,6 | / | / | / | / | 4/5 | 3 |
| Kuchuk 2015 [47] | Retrospective cohort, Canada, 2007-2008 | 383 | 116 | 5 | 13 | 302,9 | 13,1 | 43,1 | 33,9 | 112,1 | 4/5 | 3 |
| Silva 2015 [48] | Retrospective cohort, Brazil, 2007-2011 | 605 | 112 | 31 | / | 185,1 | 51,2 | 276,8 | / | / | 4/5 | 3 |
| Phanphaisarn 2016 † [27] | Retrospective cohort, Thailand, 2006-2015 | 7.455 | 797 | 68 | 56 | 106,9 | 9,1 | 85,3 | 7,5 | 70,3 | 4/5 | 3 |
| Svensson 2017 † [29] | Retrospective population based cohort, Denmark, 1994-2010. | 51.936 | 3.403 | / | / | 65,5 | / | / | / | / | Complete | 3 |
| Da Silva 2017 [49] | Retrospective cohort, Brazil, 2006-2014 | 1112 | / | 45 | / | / | 40,5 | / | / | / | 4/5 | 3 |
| Hernandez 2018 † [21] | Retrospective database, US, 2004-2013 | 59.344 | / | / | / | 129 | / | / | / | / | Complete | 4 |
| Hong 2020 † [16] | Retrospective database, Korea, 2002-2013 | 3.489 | 479 | 11 | 33 | 137,3 | 3,2 | 23,0 | 9,5 | 68,9 | 4/5 | 4 |
| Other solid tumors | ||||||||||||
| Spiegel 1995 [50] | Retrospective cohort, Germany, 1970-1991, melanoma | 7.010 | 114 | 24 | / | 16,3 | 3,4 | 210,5 | / | / | 4/5 | 3 |
| Fukutomi 2001 [51] | Retrospective cohort, Japan 1978-1997, hepatocellular carcinoma | 673 | 44 | / | / | 65,4 | / | / | / | / | Complete | 3 |
| Bhatia 2017 [52] | Retrospective cohort, US, 2005-2015, hepatocellular carcinoma | 1.017 | 14 | / | / | 13,8 | / | / | / | / | Complete | 3 |
| Harding 2018 [53] | Retrospective database, 2002-2014, US, metastatic hepatocellular carcinoma | 640 | 151 | 12 | 24 | 235,9 | 18,8 | 79,5 | 37,5 | 158,9 | 4/5 | 3 |
Table 2.
Total number of included patients, patients with MESCC, and patients with pVCF in every included RCT. SM: spinal metastases, MESCC: metastatic epidural spinal cord compression, pVCF: pathologic vertebral compression fracture. Calculated cumulative incidence of MESCC and pVCF respectively per 1000 patients with SM. Bold numbers represent the respective totals. †: datapoint not shown in Fig. 3 due to axis limits. ‡: Risk of bias: 1: properly powerd and conducted RCT, 2: well-designed controlled trial without randomization; prospective comparative cohort trial, 3: case-control studies; retrospective cohort study, 4: case series with or without intervention; cross-sectional study, 5: opinion of respected authorities, case reports.
| Author | # of patients | # of MESCC | # of pVCF | # of MESCC per 1000 SM | # of pVCF per 1000 SM | Risk of bias‡ |
|---|---|---|---|---|---|---|
| All tumors | 9594 | 371 | 1332 | 38.7 | 138.8 | |
| Rosen 2004 [54] | 773 | 25 | 127 | 32.3 | 164.3 | 1 |
| Breast carcinoma | 4454 | 104 | 786 | 23.3 | 176.5 | |
| Theriault 1999 [55] | 372 | 13 | 108 | 34.9 | 290.3 | 1 |
| Himelstein 2003 [56] | 1822 | 53 | 141 | 29.1 | 77.4 | 1 |
| Kohno 2005 [57] | 227 | 17 | 90 | 74.9 | 396.7† | 1 |
| Martin 2012 [58] | 2033 | 21 | 447 | 10.3 | 219,9 | 1 |
| Prostate carcinoma | 4264 | 238 | 416 | 55.8 | 97.6 | |
| Small 2003 [59] | 350 | 8 | 21 | 22.9 | 60.0 | 1 |
| Dearnaeley 2003 [60] | 311 | 34 | 19 | 109.3† | 61.1 | 1 |
| Saad 2004 [61] | 643 | 34 | 42 | 52.9 | 65.3 | 1 |
| Fizazi 2011 [62] | 1901 | 62 | 280 | 32.6 | 147.3 | 1 |
| Wang 2013 [63] | 137 | 2 | 7 | 14.6 | 51.1 | 1 |
| Ueno 2013 [64] | 60 | 2 | 1 | 33.3 | 16.7 | 1 |
| Pan 2014 [65] | 105 | 1 | 4 | 9.5 | 38.1 | 1 |
| James 2016 [66] | 757 | 95 | 42 | 125.5† | 55.5 | 1 |
| Lung carcinoma | ||||||
| Udagawa 2017 [67] | 103 | 4 | 3 | 38.8 | 29.1 | 3 |
3.1. Study characteristics
We retrieved three papers reporting the incidence of spinal metastases in autopsy studies. Furthermore, we retrieved three prospective and 36 retrospective clinical studies, 14 randomized controlled trials. Ten papers report data on SM, pVCF and MESCC, 12 solely on SM, 6 solely on MESCC, 1 on pVCF, 5 on SM and MESCC, 22 on MESCC and pVCF.
3.2. Quality of evidence
We evaluated all included papers by the STROBE checklist for study design, setting, participants, data sources/measurements, and study size (Table 1). All papers carefully describe their study design, setting, participants, and study size. Remarkably, none of them reports the actual definition used for MESCC or pVCF. Whereas some studies use diagnostic codes in databases [14], [15], [16], [17], [18], [19], [20], such codes refer to registrations, while being non-informative with regard to grade or severity of MESCC or pVCF. Thirty-six studies are retrospective, thus increasing the risk of bias.
3.3. Observational cohort studies
3.3.1. Incidence of spinal metastasis (SM)
Autopsy studies, performed in the 70’s and 80’s (before implementation of MRI), report SM in approximately one third of oncologic patients, none of them report the incidence of MESCC. Fornasier describes 374 autopsies of patients with malignant neoplasms (leukemia excluded) performed in the early 70’s. In 140 (37.4%) SM were detected histologically, exceeding the clinical detection with contemporary radiography (e.g. lung, breast, and prostate carcinoma histological detection rate 27%, 61%, 50%, and clinical detection rate 10%, 34%, 38% respectively) [10]. In contrast, Wong (1988) reports that not all suspected lesions (334/832, 40.1%) were histologically confirmed spinal metastases (300/832, 36.1%) [11]. Finally, Ortiz Gomez reports the presence of 225 patients (30.7%) with SM in the autopsy of 734 patients who died of malignant neoplasms between 1978 and 1987 [21]. No recent autopsy studies could be retrieved according to our search and inclusion criteria.
In observational cohort studies, mean cumulative incidence is 15.67% with a solid tumor (95% CI: 5.34-26.01) [14], [15], [18], [20], [22], [23], [24]. Three studies reported the annual incidence per 100.000 individuals, Zaikova et al. report 26.0 in South-East Norway while Choi et al. report 6.68 and Sohn et al report an increasing incidence of 6.04 to 6.83 per 100.000, in South-Korea [17], [22], [25].
Approximately two thirds of spinal metastases arise from breast (16.5%), prostate (20.7%), and lung carcinoma (24.6%) [15], [18], [23], [24], [26].
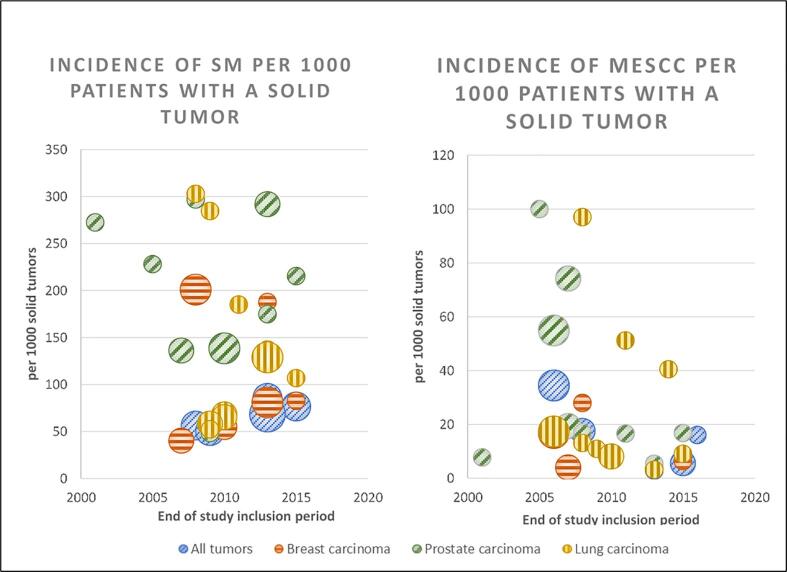
For breast carcinoma, the mean cumulative incidence of SM is 10.94% (95% CI: 5.36-16.51) [15], [18], [20], [27], [23], [24]. We excluded the incidence of SM by Domcheck et al. in this part of the analysis since they merely report the incidence of SM in patients with metastatic breast cancer and not in the entire group of breast cancer patients [29]. For prostate carcinoma, the mean cumulative incidence of SM is 20.04% (95% CI: 13.63-26.44) [15], [18], [20], [22], [23], [24], [30], [31], [32]. For lung carcinoma, it is 17.68% (95% CI: 7.44-27.92) [15], [18], [20], [22], [23], [24], [33], [34], [35], [36], [37]. (Fig. 2.A, Table 3)
Fig. 2.
Cohort studies: Incidence of SM and MESCC in relation to study period during the past two decades (2000–2020). Study period was defined by end date of study period. Size of any given circle represents study size.
Table 3.
Clinical incidence of spinal metastases (SM) in patients with solid tumors (ST) and clinical incidence of MESCC and pVCF in patients with SM of all included studies. †: The 95% confidence interval is reported In brackets †.
| Incidence of SM in patients with ST | Incidence of MESCC in patients with SM | Incidence of pVCF in patients with SM | |
|---|---|---|---|
| All solid tumors | 15.67% (5.34–26.01%)† | 9.56% (5.70%-13.42%)† | 12.63% (7.00%-18.25%)† |
| Breast carcinoma | 10.94% (5.36–16.51%)† | 6.45% (2.80%-10.09%)† | 16.55% (7.82%-25.29%)† |
| Prostate carcinoma | 20.04% (13.63–26.44%)† | 8.04% (2.09%-13.98%)† | 7.66% (5.12%-10.22%)† |
| Lung Carcinoma | 17.68% 7.44–27.92%)† | 8.27% (1.21%-15.33%)† | 8.20% (3.57%-12.83%)† |
Melanoma patients develop SM rather infrequently (merely 1.63%) [38]. Likewise, in patients with hepatocellular carcinoma (HCC), the incidence of SM is merely 1.38–6.54% [39], [40], [41].
3.3.2. Incidence of metastatic epidural spinal cord compression (MESCC)
In patients with a solid tumor, the cumulative incidence ranges from 0.29 to 10.00% (mean 2.84%, 95% CI: 1.54%-4.14%) [15], [16], [22], [24], [42], [43]. Zaikova et al. report an annual incidence of 8.1 per 100.000 inhabitants [22]. The cumulative incidence of MESCC in patients with SM, ranges from 3.41% to 36.20% (mean 19.53%, 95% CI: -6.85%-45.91%) [15], [19], [22], [24].
Mean cumulative incidence of MESCC in patients with breast, prostate or lung carcinoma is 1.47% (95% CI: 0.54%-2.40%) [15], [16], [24], [43], [28], [29], 3.63% (95% CI: 1.47%-5.78%) [15], [16], [22], [24], [43], [30], [31], [32], [45], [46], [47], [48], 2.80% (95% CI: 0.74%-4.86%) [15], [16], [22], [24], [43], [34], [35], [36] respectively and mean cumulative incidence of MESCC in patients with SM of breast, prostate, or lung carcinoma is 8.26% (95% CI: 2.28%-14.24%), 11.49% (95% CI: -2.24%-25.23%) and 8.90% (95% CI: 0.65%-17.15%) respectively in the cohort studies. (Fig. 2.B)
While included papers report no data on the incidence of MESCC in melanoma patients [38], it is reportedly 1.88% in patients with metastatic HCC [40].
3.3.3. Incidence of pathologic vertebral compression fracture (pVCF)
In patients with SM, the cumulative incidence of pVCF ranges from 5.30%-74.50% (mean 25.44%, 95% CI: -26.78%-77.66%) [15], [19], [24], [50]. Price et al. report a significantly higher incidence of 74.50% and a significant gender difference with females having an even higher incidence (81.5%) compared to males (68.0%, p<0.001), attributed to an increased prevalence of osteoporosis in female, they do comment the significantly higher incidence of pVCF overall [19]. Two papers report incidences of pVCF in patients with solid tumors, the cumulative incidence ranging from 0.41%-0.93% [15], [24]. The mean cumulative incidence of pVCF in patients with SM of breast, prostate, or lung carcinoma is 10.11% (95% CI: 6.84%-13.38%) 16,27, 32,33 , 9.62% (95% CI: 4.70%-14.54%) [15], [24], [26], [30], [32], [48] and 9.08% (95% CI: 3.98%-14.17%) [15], [24], [35], [36] respectively. While included papers report no data on the incidence of pVCF in melanoma patients, it is reported in 15.89% of patients with metastatic HCC [53]. (Fig. 2D) Wild et al. describe an important underreporting of pVCF as merely 57 out of 94 pVCFs were retained (and merely 25 were identified as an actual fracture) in radiological reports. Moreover, merely 8 were mentioned in discharge letters, and as few as 3 subsequently received any clinical management [50].
3.4. Trends over time
While few papers report the evolution of incidence rates during the study period, all of them report an increasing trend for SM or MESCC: Sohn reports a rising annual incidence from 6.04 to 6.94 per 100.000 population (p=0.01) [25] for SM. Fukutomi et al. report a threefold increase over a single decade for SM in patients with hepatocellular carcinoma [41]. Bach et al. report a rise from 4.4% to 6% of cancer patients developing MESCC during the study period (6 years) [52]. Finally Mak et al. report a significant increase in MESCC related hospitalizations at an annual rate of 6-8% [16]. Of note, because of the significant heterogeneity in included studies, we were unable to detect any significant overall trend over time (Fig. 2).
3.4.1. Randomized controlled trials
We included 14 RCTs on the effect of medication (Denosumab, Zoledronic acid, and Pamidronate) in preventing skeletal related events (SREs). Overall, these RCTs represented 9594 patients, most with breast (n=4454) or prostate (n=4264) carcinoma. While the cumulative incidence of MESCC is 3.87% in SM patients overall [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63], [64], [65], [66], those with prostate carcinoma have a higher cumulative incidence of 5.58% [58], [59], [60], [61], [62], [63], [64], [65] as compared to breast (2.33%) [54], [55], [56], [57] and lung (3.88%) [66] carcinoma. Of note, these incidences are lower than the respective incidence observed in cohort studies. Moreover, pVCF occurs more often than MESCC with an overall cumulative incidence of 13.88% of patients with SM [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63], [64], [65], [66], with breast carcinoma leading more often to pVCF (17.65%) [54], [55], [56], [57] as compared to prostate (9.76%) [58], [59], [60], [61], [62], [63], [64], [65] or lung (2.91%) [66] carcinoma.
3.4.2. Clinical incidence of MESCC and pVCF
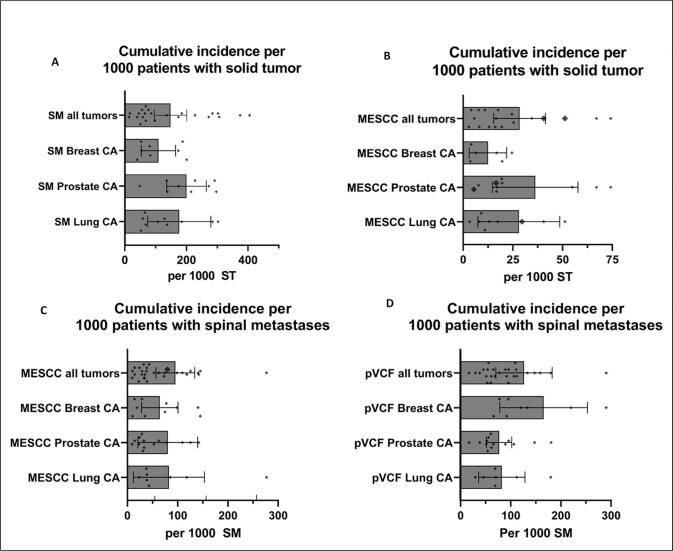
When combining all data of included RCTs and observational cohort studies we calculated a mean cumulative in patients with SM of 9.56% (95% CI 5.70%-13.42%) for MESCC and 12.63% (95% CI 7.00%-18.25%) for pVCF. The mean cumulative incidence of MESCC in patients with SM of breast, prostate, or lung carcinoma is 6.45% (95% CI: 2.80%-10.09%), 8.04% (95% CI: 2.09%-13.98%) and 8.27% (95% CI: 1.21%-15.33%) respectively. The mean cumulative incidence of pVCF in patients with SM of breast, prostate, or lung carcinoma 16.55% (95% CI: 7.82%-25.29%), 7.66% (95% CI: 5.12%-10.22%) and 8.20% (95% CI: 3.57%-12.83%) respectively (Fig. 3, Table 3).
Fig. 3.
Cumulative incidence of spinal metastases (SM), Metastatic Epidural Spinal Cord Compression (MESCC) and pathologic Vertebral Compression Fractures (pVCF). CA = carcinoma, ST = solid tumor. Solid bars represent the mean values and error bars represent the 95% CI. While some higher values are not shown in the graphs due to axis limits, these values are included in both mean and 95% CI error bars and can be found in Table 1, Table 2.
3.4.3. Time to develop SM, time from SM to MESCC, time from MESCC to loss of function
Hong et al. report that patients with lung cancer have a shortest interval of approximately 9.0 months, while those with breast and prostate carcinoma have a significantly longer interval of 14.9 and 17.4 months. This difference in interval is confirmed by Svensson et al. (median time to develop SM 27.9-29.5 months for lung carcinoma compared to 3.5-4 years for breast carcinoma) and Bach et al. describes comparable difference for MESCC (0.5 years for lung carcinoma compared to 4.6 years for breast - and 1.7 years for prostate carcinoma) [15], [23], [52]. In line with these observations, Hernandez et al. report a one and 10 year incidence of bone metastases for breast, prostate, and lung carcinoma of 3.4% and 8.1%, 18.0% and 29.2%, and 10.45% and 12.9% respectively [20]. Interestingly, most pVCF and MESCC develop within one month after diagnosing bone metastasis except for patients with breast and prostate carcinoma who tend to have a slightly longer interval (median 5.9 and 4.7 months respectively) [15].
Few papers report the incidence of MESCC and motor deficit separately. Interestingly, they all demonstrate that approximately one third of MESCC patients have an actual neurological deficit (31% MESCC and 11% paresis [22], 36.2% MESCC and 9.5% paresis [19] , 27.7% MESCC and 12,5% unable to walk [33], 45 patients with MESCC and 15 unable to walk49).
Venkitaraman et al. report the time to develop a neurologic deficit in patients with subclinical radiographic MESCC (rMESCC). They observed a mean of 657 days (95%CI: 23-1103 days) if rMESCC was present on initial MRI. In case it was not, 21.5 % of patients ultimately did develop rMESCC with a median interval of 283 days (95%CI: 229-337). Of note, in prostate cancer, a rapid Prostaste-Specific Antigen doubling time has been demonstrated to be a statistically significant independent predictor for developing a neurological deficit (p=0.042) [31]. The proportion of patients with MESCC on initial MRI (n=37) free from neurological deficit at 3, 6, 12, 18, and 24 months was 94, 80, 59, 59 and 43% respectively. [31] No other included studies report the time to develop a neurologic deficit.
Pamidronate, Zoledronic acid and Denosumab are able to prolong the time to first skeletal complication. Moreover these medications are able to reduce the incidence of skeletal complications in patients with bone metastases [53], [55], [56], [57], [58], [61], [62], [63], [64].
3.4.4. Symptomatology
Bach et al. elaborately report the clinical presentation of 398 patients with MESCC. The majority of patients (83%) presented with pain (47% radicular pain and 36% local back pain). Ataxia (67%) was more common compared to weakness (27%) as initial symptom. Almost half had severe sphincter disturbances with 47% being catheter dependent and 18% having moderate symptoms. Mean duration from first symptoms to diagnosis of MESCC was 58 days (average 30 days, range 0-420 days) [52].
3.4.5. Survival
Patients with SM have an overall median survival of 6 months [24], with longer median survival times for breast (11.1-22 months) [15], [24], [29] and prostate (16-38.1 months) [15], [24] carcinoma as compared to lung carcinoma (2.8-5.8 months) [15], [24], [33], [34], [51]. This trend is confirmed by studies reporting the 1y survival rate overall (31.5%) [24] and for breast, prostate, and lung carcinoma being 48.3-66.3% [18], [23], [24], [28], 35-87.0% [18], [23], [24], [30] , and 10.6-22% [18], [23], [24], [36], [51] respectively.
For patients with MESCC, an overall median survival of 2.9-3.1 months [42], [43], [52]is reported, with longer median survival times for breast (5.0 months) and prostate (4.0 months) carcinoma [43] as compared to lung carcinoma (1.5-2.8 months 33,43). MESCC is associated with an increased risk of death (Hazard ratio 1.62 (95% CI: 1.18-2.23)) [49]. The development of paralysis is associated with a significant decrease in survival (1y survival 17.6% as compared to 96.6%) [28].
While some studies suggest an increase in survival with Pamidronate, Zoledronic acid or Denosumab treatment [65], [66], most studies have failed to demonstrate such benefit [56], [58], [60], [63].
4. Discussion
4.1. Incidence of SM, MESCC and pVCF
The most frequently cited incidence of SM of 30% is based on autopsy studies, in contrast, the clinical observations included in this review estimate that 15,67% of patients with solid tumors develop clinical diagnosed SM and 2.8% will eventually develop MESCC (Fig. 3A, B). Roughly 1 in 10 patients with SM will develop MESCC (9.5%) or pVCF (12.6%) (Fig. 3C, D). Breast, prostate, and lung carcinoma are the most prevalent solid tumors to develop SM, with a respective cumulative incidence of 10.94%, 20.04% and 17.68% SM in cohort studies. Studies showed that there is an important delay between the development of symptoms and diagnosis of more than two months [52], [67]. This delay is occasionally or partially explained by late presentation of patients, nonetheless delayed diagnosis by clinicians occurs to frequently [52], [67]. This high clinical and even higher histologic incidence in autopsy studies should encourage clinicians to be aware of signs and symptoms suggestive of SM, MESCC and pVCF in every patients with a solid tumor, since a delayed diagnosis may have a profound impact on both QOL and LOL.
4.2. Timely diagnosis of SM, MESCC and pVCF
Back pain is the most common initial symptom of SM and may be observed in as many as 88-94% of patients at time of diagnosis. MESCC may lead to more specific symptoms including radicular pain (50% as initial symptom and close to 80% at diagnosis). Ataxia (67%) is a more frequently presenting symptom compared to motor weakness (40% as initial symptom and close to 90% at diagnosis) and finally sensory disturbances (30% to 75%) and/or bladder dysfunction (5 to 60% at diagnosis)may be one of the presenting symptoms of MESCC [52], [68], [69]. Often symptoms are slowly progressive and patients may only present when their mobility is affected despite experiencing symptoms for several weeks or even months [67]. Nonetheless delayed diagnosis of symptomatic patients by clinicians seems to occur frequently [52], [67]. This leads to delay in treatment with forthcoming negative impact on QOL and LOL. In oncologic patients with back pain (suggesting SM), radicular pain, motor weakness, sensory complaints and/or bladder dysfunction (suggesting MESCC), a careful and timely diagnostic work up is mandatory in order to avoid delayed diagnosis, that would increases the odds of a persisting neurological deficit and resulting loss of ambulation, which in turn would reduce QOL and LOL [6], [7], [67], [69].
4.3. MESCC and neurological deficit
There are no reliable models to predict if or when neurologic deficit might occur. Different studies have demonstrated that approximately one third of patients with MESCC will eventually develop a neurological deficit and resulting inability to walk [19], [22], [33], [49]. Venkitaraman et al. demonstrated that the proportion of patients with MESCC free from neurological deficit declines in time [31]. As expected, these numbers suggest the natural history of SM is progressive and most if not all patients with occult MESCC on screening MRI, if untreated, will eventually develop a neurological deficit if survival permits.
4.4. (The problem with) predictive survival scores
Survival of patients with SM from solid tumors has improved, as confirmed by recent studies by Tabourel et al. [70] and Carrwik et al. [71], who report an actual underestimation of by commonly used predictive survival scores including the revised Tokuhashi, Tomita, modified Bauer, Lei, Van der Linden, and Rades score. Predictive accuracy of individual scores ranges from 25.6 to 61.0% [70], far worse than predictive accuracy in previously published validation studies, a difference largely explained by underestimating survival. This is explained by significant improvements in oncologic treatments and resulting increased life expectancy over the past decades [2]. In daily clinical practice, underestimating actual survival may be a trigger to withhold treatment to selected patients. Clearly, the decision whether or not to treat should no longer be based solely on these prognostic scores. In this analysis we were unable to demonstrate any significant change in survival rates due to the heterogenous study populations, the range of retrospective studies over relatively long study periods, and an overall lack of detailed survival data. Despite an improved overall survival, the presence of SM still has an important negative influence on actual survival with a reported hazard ratio risk of death of 1.62 [49], a 40% decrease in 1-year survival rate for patients with prostate carcinoma and SM as compared to those without SM, and an even further decrease if pVCF or MESCC are present [30].
4.5. SM, MESCC and pVCF: where do we stand?
Due to the increasing incidence of solid tumors and improving survival of these patients, more patients are at risk to develop SM, MESCC and pVCF. The incidence of SM, MESCC and pVCF is not changing significantly over time for patients with a solid tumor and the survival of those patients is improving. Hereby, one may conclude that SM, MESCC, and pVCF are increasingly prevalent.
Over the past decade radiotherapy has drastically improved. The introduction of stereotactic body radiotherapy (SBRT) in the treatment of SM has led to a significant improvement in local tumor control and pain control without a significant difference in toxicity [72]. A recent systematic review and meta-analysis of de novo SM demonstrates that single fraction stereotactic radiosurgery (SF-SRS) is superior in local control as compared to conventional radiotherapy (RT) with 1-year local control (LC) of 95.5% (95%CI: 87.4-99.6%) as compared to 83.6% (95%CI: 69.2-90.5%) [73]. SBRT has a significant benefit in pain response with minimal toxicity [72], [73], [74].
In the meantime, surgery is shifting towards less invasive treatment with a growing interest in so-called separation surgery for MESCC. These less invasive techniques are associated with reduced intra operative bleeding, reduced surgical trauma, fewer complications and a shorter length of in-hospital stay [75].
While prompt treatment of MESCC is associated with increased odds of recovery once a neurological deficit develops, treatment before a significant deficit occurs is clearly associated with increased odds of remaining neurologically intact [6], [7]. Surgery followed by adjuvant conventional radiotherapy is considered superior to merely conventional radiotherapy, with preserved ambulatory function in 84% and 54% respectively (OR 6.2, p=0.001), and significantly longer preservation of ambulatory function (median 122 and 13 days respectively, p=0.003) [8]. In the subgroup of ambulatory patients with MESCC, 94% remained ambulatory for a median of 155 days after decompressive surgery as compared to 74% for a median of 54 days (OR 1.82, p=0.024) in the conventional radiotherapy group. [8] As RCTs with regard to this subject are scarce, it is still unclear which treatment or combination of treatments is superior when comparing more recently improved modalities in radiotherapy and surgery. While separation surgery followed by SBRT has proven to be effective and is gaining interest, there are no RCTs thus far to support this trend. Continued prospective, randomized research is warranted to develop and validate optimal radiotherapeutic regimens and to define optimal surgical indications and strategies.
4.6. Study limitations
This review has some limitations. First, the selection and/or registration bias in included papers, because most are not population based and many are retrospective, the cohort studies are based on different populations and a bias may exist if some of these studies are performed in tertiary oncologic centers. Second, different definitions or cut-offs are likely used to define MESCC, (e.g. every radiological evidence for SM or MESCC or merely clinically symptomatic SM or MESCC), while none of the included papers report the exact definition they used for MESCC. All this, unfortunately, leads to a range in reported incidences of SM and MESCC. This heterogeneity with lack of definitions makes it impossible to perform a random-effects meta-analysis. In this regard, Bilsky et al. suggested a grading system for MESCC . Clearly, defining the in- and exclusion criteria for MESCC is important to ensure validity of study results when applied to an individual patient in daily clinical practice. Third, there was insufficient data to perform in detail analysis for all different tumor types, therefore we limited the analysis to overall and to the most prevalent subgroups of breast-, prostate and lung carcinoma.
5. Conclusion
The combination of a growing incidence of cancer and improving survival rates, generates more patients at risk to develop SM, MESCC and pVCF. While the overall cumulative incidence for clinically diagnosed SM in living patients is reportedly 15.67%, autopsy studies reveal that SM are actually present in as many as 30% of patients with solid tumor by the time they die, suggesting underdiagnosing of SM. Approximately 1 out of ten patients with SM from a solid tumor will develop MESCC (9.5%), and another 12.6% will develop a pVCF. Studies show an important delay between onset of symptoms and diagnosis. Understanding these epidemiologic data, should increase awareness for first symptoms, allowing early diagnosis and subsequent treatment, thus improving overall outcome.
Funding
This research received no external financial or non-financial support. There are no additional relationships to disclose. There are no patents to disclose. There are no additional activities to disclose.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.World Health Organization. Who Report on Cancer: Setting Priorities, Investing Wesely and Providing Care for All.; 2020.
- 2.Allemani C., Matsuda T., di Carlo V., et al. Global surveillance of trends in cancer survival: analysis of individual records for 37,513,025 patients diagnosed with one of 18 cncers during 2000–2014 from 322 populatio-based registries in 71 countries (CONCORD-3) Lancet. 2018;391(10125):1023–1075. doi: 10.1016/S0140-6736(17)33326-3.Global. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kypriotakis G., Vidrine D.J., Francis L.E., Rose J.H. The longitudinal relationship between quality of life and survival in advanced stage cancer. Psychooncology. 2016;25(2):225–231. doi: 10.1002/pon.3846.The. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ediebah D.E., Coens C., Zikos E., et al. Does change in health-related quality of life score predict survival? Analysis of EORTC 08975 lung cancer trial. Br. J. Cancer. 2014;110(10):2427–2433. doi: 10.1038/bjc.2014.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shrestha A., Martin C., Burton M., Walters S., Collins K., Wyld L. Quality of life versus length of life considerations in cancer patients: A systematic literature review. Psycho-Oncology. 2019;28(7):1367–1380. doi: 10.1002/pon.5054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu Y.H., Hu Y.C., Yang X.G., et al. Prognostic factors of ambulatory status for patients with metastatic spinal cord compression: a systematic review and meta-analysis. World Neurosurg. 2018;116 doi: 10.1016/j.wneu.2018.04.188. e278 e290. [DOI] [PubMed] [Google Scholar]
- 7.Quraishi N.A., Rajagopal T.S., Manoharan S.R., Elsayed S., Edwards K.L., Boszczyk B.M. Effect of timing of surgery on neurological outcome and survival in metastatic spinal cord compression. Eur. Spine J. 2013;22:1383–1388. doi: 10.1007/s00586-012-2635-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patchell R.A., Tibbs P.A., Regine W.F., et al. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: a randomised trial. Lancet. 2005;366:643–648. doi: 10.1016/s0140-6736(05)66954-1. [DOI] [PubMed] [Google Scholar]
- 9.Fisher CG, Dipaola CP, Ryken TC, et al. A novel classification system for spinal instability in neoplastic disease: An evidence-based approach and expert consensus from the spine oncology study group. Spine (Phila Pa 1976). 2010;35(22):1221-12210.1097/BRS.0b013e3181e16ae2. [DOI] [PubMed]
- 10.Fornasier V.L., Horne J.G. Metastases to the vertebral column. Cancer. 1975;36(2):590–594. doi: 10.1002/1097-0142(197508)36:2<590::AID-CNCR2820360240>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 11.Wong DA, Fornasier VL, MacNab I. Spinal Metastases: The obvious, the occult and the impostors. Spine (Phila Pa 1976). 1990;15(1):1-4. http://www.ncbi.nlm.nih.gov/pubmed/?term=spinal+metastases:+the+obvious,+the+occult+and+the+imposters. [PubMed]
- 12.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. The BMJ. 2021;372. 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed]
- 13.von Elm E., Altman D., Egger M., et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE)statement: guidelines for reporting observational studies. J. Clin. Epidemiol. 2008;61(4):344–349. doi: 10.1016/j.jclinepi.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 14.Schulman K.L., Kohles J. Economic burden of metastatic bone disease in the U.S. Cancer. 2007;109(11):2334–2342. doi: 10.1002/cncr.22678. [DOI] [PubMed] [Google Scholar]
- 15.Hong S., Youk T., Lee S.J., Kim K.M., Vajdic C.M. Bone metastasis and skeletal-related events in patients with solid cancer: A Korean nationwide health insurance database study. PLoS ONE. 2020;15(7):1–13. doi: 10.1371/journal.pone.0234927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mak K.S., Lee L.K., Mak R.H., et al. Incidence and treatment patterns in hospitalizations for malignant spinal cord compression in the United States, 1998–2006. Int. J. Radiat. Oncol. Biol. Phys. 2011;80(3):824–831. doi: 10.1016/j.ijrobp.2010.03.022. [DOI] [PubMed] [Google Scholar]
- 17.Choi SH, Koo JW, Choe D, Kang CN. The Incidence and Management Trends of Metastatic Spinal Tumors in South Korea: A Nationwide Population-based Study. Spine (Phila Pa 1976). 2020;45(14):E856-E863. 10.1097/BRS.0000000000003445. [DOI] [PubMed]
- 18.Oster G., Lamerato L., Glass A.G., et al. Natural history of skeletal-related events in patients with breast, lung, or prostate cancer and metastases to bone: A 15-year study in two large US health systems. Support. Care Cancer. 2013;21(12):3279–3286. doi: 10.1007/s00520-013-1887-3. [DOI] [PubMed] [Google Scholar]
- 19.Price M., Goodwin J.C., de la Garza R.R., et al. Gender disparities in clinical presentation, treatment, and outcomes in metastatic spine disease. Cancer Epidemiol. 2021;70 doi: 10.1016/j.canep.2020.101856. [DOI] [PubMed] [Google Scholar]
- 20.Hernandez R.K., Wade S.W., Reich A., Pirolli M., Liede A., Lyman G.H. Incidence of bone metastases in patients with solid tumors: Analysis of oncology electronic medical records in the United States. BMC Cancer. 2018;18(1) doi: 10.1186/s12885-017-3922-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gomez J.A.O. The incidence of vertebral body metastases. Int. Orthop. 1995;19(5):309–311. doi: 10.1007/BF00181116. http://link.springer.com/article/10.1007/BF00181116 [DOI] [PubMed] [Google Scholar]
- 22.Zaikova O., Giercksky K., Fossa S.D., Kvaløy S., Johannesen T.B., Skjeldal S. A population-based study of spinal metastatic disease in south-east norway. Clin. Oncol. 2009;21:753–759. doi: 10.1016/j.clon.2009.09.023. [DOI] [PubMed] [Google Scholar]
- 23.Svensson E., Christiansen C.F., Ulrichsen S.P., Rørth M.R., Sørensen H.T. Survival after bone metastasis by primary cancer type: a Danish population-based cohort study. BMJ Open. 2017;7(9):1–7. doi: 10.1136/bmjopen-2017-016022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Phanphaisarn A., Patumanond J., Settakorn J., Chaiyawat P., Klangjorhor J., Pruksakorn D. Prevalence and survival patterns of patients with bone metastasis from common cancers in Thailand. Asian Pac. J. Cancer Prev. 2016;17(9):4335–4340. [PubMed] [Google Scholar]
- 25.Sohn S., Kim J., Chung C.K., et al. Nationwide epidemiology and healthcare utilization of spine tumor patients in the adult Korean population, 2009–2012. Neuro-Oncol. Pract. 2015;2(2):93–100. doi: 10.1093/nop/npv006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baek Y.H., Jeon H.L., Oh I.S., Yang H., Park J., Shin J.Y. Incidence of skeletal-related events in patients with breast or prostate cancer-induced bone metastasis or multiple myeloma: A 12-year longitudinal nationwide healthcare database study. Cancer Epidemiol. 2019;61:104–110. doi: 10.1016/j.canep.2019.05.013. [DOI] [PubMed] [Google Scholar]
- 27.Jensen A.T., Jacobsen J.B., Nørgaard M., Yong M., Fryzek J.P., Sørensen H.T. Incidence of bone metastases and skeletal-related events in breast cancer patients: a population-based cohort study in Denmark. BMC Cancer. 2011;11:2–7. doi: 10.1186/1471-2407-11-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oka H., Kondoh T., Seichi A., Hozumi T., Nakamura K. Incidence and prognostic factors of Japanese breast cancer patients with bone metastasis. J. Orthopaedic Sci. 2006;11(1):13–19. doi: 10.1007/s00776-005-0966-9. [DOI] [PubMed] [Google Scholar]
- 29.Domchek S.M., Younger J., Finkelstein D.M., Seiden M.V. Predictors of skeletal complications patients with breast carcinoma. Cancer. 2000;89(2):363–368. doi: 10.1002/1097-0142(20000715)89:2<363::AID-CNCR22>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 30.Nørgaard M, Østergaard Jensen A, Jacobsen JB, Cetin K, Fryzek JP, SØrensen HT. Skeletal Related Events, Bone Metastasis and Survival of Prostate Cancer: A Population Based Cohort Study in Denmark (1999 to 2007). The Journal of Urology. 2010;184:162-167. [DOI] [PubMed]
- 31.Venkitaraman R., Sohaib S.A., Barbachano Y., et al. Frequency of screening magnetic resonance imaging to detect occult spinal cord compromise and to prevent neurological deficit in metastatic castration-resistant prostate cancer. Clin. Oncol. 2010;22(2):147–152. doi: 10.1016/j.clon.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 32.Perrault L., Fradet V., Lauzon V., Lelorier J., Mitchell D., Habib M. Burden of illness of bone metastases in prostate cancer patients in Québec, Canada: a population-based analysis. J. Can. Urol. Assoc. 2015;9:307–314. doi: 10.5489/cuaj.2707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Silva G.T., Bergmann A., Thuler L.C.S. Incidence, associated factors, and survival in metastatic spinal cord compression secondary to lung cancer. Spine J. 2015;15(6):1263–1269. doi: 10.1016/j.spinee.2015.02.015. [DOI] [PubMed] [Google Scholar]
- 34.Kuchuk M., Kuchuk I., Sabri E., Hutton B., Clemons M., Wheatley-Price P. The incidence and clinical impact of bone metastases in non-small cell lung cancer. Lung Cancer. 2015;89(2):197–202. doi: 10.1016/j.lungcan.2015.04.007. [DOI] [PubMed] [Google Scholar]
- 35.Katakami N., Kunikane H., Takeda K., et al. Prospective study on the incidence of infection by. J. Clin. Med. 2014;9(2):231–238. doi: 10.1097/JTO.0000000000000051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cetin K., Christiansen C.F., Jacobsen J.B., Nørgaard M., Sørensen H.T. Bone metastasis, skeletal-related events, and mortality in lung cancer patients: a Danish population-based cohort study. Lung Cancer. 2014;86(2):247–254. doi: 10.1016/j.lungcan.2014.08.022. [DOI] [PubMed] [Google Scholar]
- 37.Dalgaard K.S., Gammelager H., Sværke C., Kurics T., Cetin K., Christiansen C.F. Hospital use among patients with lung cancer complicated by bone metastases and skeletal-related events: a population-based cohort study in Denmark. Clin. Epidemiol. 2015;7:363–368. doi: 10.2147/CLEP.S78301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Spiegel DA, Sampson JH, Richardson WJ, et al. Metastatic Melanoma to the Spine: Demographics, Risk Factors, and prognosis in 114 patients. Spine (Phila Pa 1976). 1995;20(19):2141-2146. [DOI] [PubMed]
- 39.Bhatia R., Ravulapati S., Befeler A., Dombrowski J., Gadani S., Poddar N. hepatocellular carcinoma with bone metastases: incidence, prognostic significance, and management—single-center experience. J. Gastroint. Cancer. 2017;48(4):321–325. doi: 10.1007/s12029-017-9998-6. [DOI] [PubMed] [Google Scholar]
- 40.Harding J.J., Abu-Zeinah G., Chou J.F., et al. Frequency, morbidity, and mortality of bone metastases in advanced hepatocellular carcinoma. JNCCN J. Natl. Comprehen. Cancer Netw. 2018;16(1):50–58. doi: 10.6004/jnccn.2017.7024. [DOI] [PubMed] [Google Scholar]
- 41.Fukutomi M., Yokota M., Chuman H., et al. Increased incidence of bone metastases in hepatocellular carcinoma. Eur. J. Gastroenterol. Hepatol. 2001;13(9):1083–1088. doi: 10.1097/00042737-200109000-00015. [DOI] [PubMed] [Google Scholar]
- 42.Campillo-Recio D., Jimeno Ariztia M., Flox Benítez G., Marco Martínez J., Vicente Martín C., Plaza C.S. Metastatic spinal cord compression: Incidence, epidemiology and prognostic factors. Rev. Clín. Española (English Ed.) 2019;219(7):386–389. doi: 10.1016/j.rceng.2019.03.010. [DOI] [PubMed] [Google Scholar]
- 43.Loblaw D.A., Laperriere N.J., Mackillop W.J. A population-based study of malignant spinal cord compression in Ontario. Clin. Oncol. 2003;15(4):211–217. doi: 10.1016/S0936-6555(02)00400-4. [DOI] [PubMed] [Google Scholar]
- 44.Yong M., Jensen A.Ø., Jacobsen J.B., Nørgaard M., Fryzek J.P., Sørensen H.T. Survival in breast cancer patients with bone metastases and skeletal-related events: a population-based cohort study in Denmark (1999–2007) Breast Cancer Res. Treat. 2011;129(2):495–503. doi: 10.1007/s10549-011-1475-5. [DOI] [PubMed] [Google Scholar]
- 45.Kuban D.A., El-mahdi A.M., Sigfred S.V., Schellhammer P.F., Babb T.J. Characteristics of spinal cord compression in adenocarcinoma of prostate. Urology. 1986;28(5):364–369. doi: 10.1016/0090-4295(86)90062-2. [DOI] [PubMed] [Google Scholar]
- 46.Kawai A.T., Martinez D., Saltus C.W., Vassilev Z.P., Soriano-Gabarró M., Kaye J.A. Incidence of second primary malignancies in patients with castration-resistant prostate cancer: an observational retrospective cohort study in the United States. Prostate Cancer. 2019;2019 doi: 10.1155/2019/4387415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Onukwugha E., Yong C., Mullins C.D., Seal B., McNally D., Hussain A. Skeletal-related events and mortality among older men with advanced prostate cancer. J. Geriat. Oncol. 2014;5(3):281–289. doi: 10.1016/j.jgo.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 48.Berruti A., Dogliotti L., Bitossi R., et al. Incidence of skeletal complications in patients with bone metastatic prostate cancer and hormone refractory disease: predictive role of bone resorption and formation markers evaluated at baseline. J. Urol. 2000;164(4):1248–1253. doi: 10.1016/S0022-5347(05)67149-2. [DOI] [PubMed] [Google Scholar]
- 49.da Silva G.T., Bergmann A., Thuler L.C.S. Impact of symptomatic metastatic spinal cord compression on survival of patients with non-small-cell lung cancer. World Neurosurgery. 2017;108:698–704. doi: 10.1016/j.wneu.2017.09.079. [DOI] [PubMed] [Google Scholar]
- 50.Wild M., Dankerl P., Hammon M., Uder M., Janka R. Vertebral body fractures of unknown origin in cancer patients receiving MDCT: reporting by radiologists and awareness by clinicians. Springerplus. 2016;5(1) doi: 10.1186/s40064-016-2097-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Decroisette C., Monnet I., Berard H., et al. Epidemiology and treatment costs of bone metastases from lung cancer: A French prospective, observational, multicenter study (GFPC 0601) J. Thor. Oncol. 2011;6(3):576–582. doi: 10.1097/JTO.0b013e318206a1e3. [DOI] [PubMed] [Google Scholar]
- 52.Bach F., Larsen B.H., Rohde K., et al. Metastatic spinal cord compression - Occurrence, symptoms, clinical presentations and prognosis in 398 patients with spinal cord compression. Acta Neurochir. 1990;107(1–2):37–43. doi: 10.1007/BF01402610. [DOI] [PubMed] [Google Scholar]
- 53.Rosen L.S., Gordon D., Tchekmedyian N.S., et al. Long-term efficacy and safety of zoledronic acid in the treatment of skeletal metastases in patients with nonsmall cell lung carcinoma and other solid tumors: a randomized, phase III, double-blind, placebo-controlled trial. Cancer. 2004;100(12):2613–2621. doi: 10.1002/cncr.20308. [DOI] [PubMed] [Google Scholar]
- 54.Himelstein A.L., Foster J.C., Khatcheressian J.L., et al. Effect of longer-interval vs standard dosing of zoledronic acid on skeletal events in patients with bone metastases: a randomized clinical trial. JAMA Oncol. 2017;317(1):48–58. doi: 10.1001/jama.2016.19425.Effect. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kohno N., Aogi K., Minami H., et al. Zoledronic acid significantly reduces skeletal complications compared with placebo in Japanese women with bone metastases from breast cancer: a randomized, placebo-controlled trial. J. Clin. Oncol. 2005;23(15):3314–3321. doi: 10.1200/JCO.2005.05.116. [DOI] [PubMed] [Google Scholar]
- 56.Theriault R.L., Lipton A., Hortobagyi G.N., et al. Pamidronate reduces skeletal morbidity in women with advanced breast cancer and lytic bone lesions: a randomized Placebo-Controlled Trial. J. Clin. Oncol. 1999;17(3):846–854. doi: 10.1200/JCO.1999.17.3.846. [DOI] [PubMed] [Google Scholar]
- 57.Martin M., Bell R., Bourgeois H., et al. Bone-related complications and quality of life in advanced breast cancer: Results from a randomized phase III trial of denosumab versus zoledronic acid. Clin. Cancer Res. 2012;18(17):4841–4849. doi: 10.1158/1078-0432.CCR-11-3310. [DOI] [PubMed] [Google Scholar]
- 58.James N.D., Pirrie S.J., Pope A.M., et al. Clinical outcomes and survival following treatment of metastatic castrate-refractory prostate cancer with docetaxel alone or with strontium-89, zoledronic acid, or both: The TRAPEZE randomized clinical trial. JAMA Oncol. 2016;2(4):493–499. doi: 10.1001/jamaoncol.2015.5570. [DOI] [PubMed] [Google Scholar]
- 59.Small E.J., Smith M.R., Seaman J.J., Petrone S., Kowalski M.O. Combined analysis of two multicenter, randomized, placebo-controlled studies of pamidronate disodium for the palliation of bone pain in men with metastatic prostate cancer. J. Clin. Oncol. 2003;21(23):4277–4284. doi: 10.1200/JCO.2003.05.147. [DOI] [PubMed] [Google Scholar]
- 60.Dearnaley D.P., Sydes M.R., Mason M.D., et al. A double-blind, placebo-controlled, randomized trial of oral sodium clodronate for metastatic prostate cancer (MRC PR05 Trial) J. Natl. Cancer Inst. 2003;95(17):1300–1311. doi: 10.1093/jnci/djg038. [DOI] [PubMed] [Google Scholar]
- 61.Saad F., Gleason D.M., Murray R., et al. Long-term efficacy of zoledronic acid for the prevention of skeletal complications in patients with metastatic hormone-refractory prostate cancer. J. Natl Cancer Inst. 2004;96(11):879–882. doi: 10.1093/jnci/djh141. [DOI] [PubMed] [Google Scholar]
- 62.Fizazi K., Carducci M., Smith M., et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a randomised, double-blind study. Lancet. 2011;377(9768):813–822. doi: 10.1016/S0140-6736(10)62344-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang F., Chen W., Chen H., et al. Comparison between zoledronic acid and clodronate in the treatment of prostate cancer patients with bone metastases. Med. Oncol. 2013;30(3) doi: 10.1007/s12032-013-0657-x. [DOI] [PubMed] [Google Scholar]
- 64.Ueno S., Mizokami A., Fukagai T., et al. Efficacy of combined androgen blockade with zoledronic acid treatment in prostate cancer with bone metastasis: the ZABTON-PC (Zoledronic Acid/androgen bloackade trial on prostate cancer) study. Anticancer Res. 2003;33:3837–3844. [PubMed] [Google Scholar]
- 65.Pan Y., Jin H., Chen W., et al. Docetaxel with or without zoledronic acid for castration-resistant prostate cancer. Int. Urol. Nephrol. 2014;46(12):2319–2326. doi: 10.1007/s11255-014-0824-9. [DOI] [PubMed] [Google Scholar]
- 66.Udagawa H., Niho S., Kirita K., et al. Impact of denosumab use on the survival of untreated non-squamous non-small cell lung cancer patients with bone metastases. J. Cancer Res. Clin. Oncol. 2017;143(6):1075–1082. doi: 10.1007/s00432-017-2350-5. [DOI] [PubMed] [Google Scholar]
- 67.Shah S., Kutka M., Lees K., et al. Management of metastatic spinal cord compression in secondary care: a practice reflection from medway maritime hospital, Kent, UK. J. Personal. Med. 2021;11(2):1–12. doi: 10.3390/jpm11020110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Helweg-Larsen S., Sørensen P.S. Symptoms and signs in metastatic spinal cord compression: a study of progression from first symptom until diagnosis in 153 patients. Eur. J. Cancer. 1994;30(3):396–398. doi: 10.1016/0959-8049(94)90263-1. [DOI] [PubMed] [Google Scholar]
- 69.Levack P., Graham J., Collie D., et al. Don’t wait for a sensory level – Listen to the symptoms: a prospective audit of the delays in diagnosis of malignant cord compression. Clin. Oncol. 2002;14(6):472–480. doi: 10.1053/clon.2002.0098. [DOI] [PubMed] [Google Scholar]
- 70.Tabourel G., Terrier L.M., Dubory A., et al. Are spine metastasis survival scoring systems outdated and do they underestimate life expectancy? Caution in surgical recommendation guidance. J. Neurosurg. Spine. 2021;35(October):1–8. doi: 10.3171/2020.12.spine201741. [DOI] [PubMed] [Google Scholar]
- 71.Carrwik C, Olerud C, Robinson Y. Predictive Scores Underestimate Survival of Patients with Metastatic Spine Disease: A Retrospective Study of 315 Patients in Sweden. Spine (Phila Pa 1976). 2020;45(6):414-419. 10.1097/BRS.0000000000003289. [DOI] [PubMed]
- 72.Billiet C., Joye I., Mercier C., et al. Outcome and toxicity of hypofractionated image-guided SABR for spinal oligometastases. Clin. Transl. Radiat. Oncol. 2020;24:65–70. doi: 10.1016/j.ctro.2020.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Singh R., Lehrer E.J., Dahshan B., et al. Single fraction radiosurgery, fractionated radiosurgery, and conventional radiotherapy for spinal oligometastasis (SAFFRON): a systematic review and meta-analysis. Radiother. Oncol. 2020;146:76–89. doi: 10.1016/j.radonc.2020.01.030. [DOI] [PubMed] [Google Scholar]
- 74.Sahgal A., Myrehaug S.D., Siva S., et al. Stereotactic body radiotherapy versus conventional external beam radiotherapy in patients with painful spinal metastases: an open-label, multicentre, randomised, controlled, phase 2/3 trial. Lancet Oncol. 2021;22(7):1023–1033. doi: 10.1016/S1470-2045(21)00196-0. [DOI] [PubMed] [Google Scholar]
- 75.Hinojosa-Gonzalez DE, Roblesgil-Medrano A, Villarreal-Espinosa JB, et al. Minimally Invasive versus Open Surgery for Spinal Metastasis: A Systematic Review and Meta-Analysis. Asian Spine Journal. Published online 2021. 10.31616/asj.2020.0637. [DOI] [PMC free article] [PubMed]