Abstract
Background
Because of their well-described immunosuppressive properties, allogeneic adult human mesenchymal stromal cells (MSC) derived from bone marrow have demonstrated safety and efficacy in steroid refractory acute graft versus host disease (SR aGVHD). Clinical trials have resulted in variable success and an optimal source of MSC has yet to be defined. Based on the importance of maternal-fetal interface immune tolerance, extraembryonic fetal tissues, such as the umbilical cord, may provide an superior tissue source of MSC to mediate immunomodulation in aGVHD.
Methods
A two-dose cohort trial allogeneic Wharton’s Jelly-derived mesenchymal stromal cells (WJMSC, referred to as MSCTC-0010, here) were tested in 10 patients with de novo high risk (HR) or SR aGVHD post allogeneic hematopoietic stem cell transplantation (allo-HCT). Following Good Manufacturing Practices isolation, expansion and cryostorage, WJMSC were thawed and administered via intravenous infusions on days 0 and 7 at one of two doses (low dose cohort, 2×106/kg, n=5; high dose cohort, 10×106/kg, n=5). To evaluate safety, patients were monitored for infusion related toxicity, Treatment Related Adverse Events (TRAE) til day 42, or ectopic tissue formation at day 90. Clinical responses were monitored at time points up to 180 days post infusion. Serum biomarkers ST2 and REG3α were acquired 1 day prior to first MSCTC-0010 infusion and on day 14.
Results
Safety was indicated, e.g., no infusion-related toxicity, no development of TRAE, nor ectopic tissue formation in either low or high dose cohort was observed. Clinical response was suggested at day 28: the overall response rate (ORR) was 70%, 4 of 10 patients had a complete response (CR) and 3 had a partial response (PR). By study day 90, the addition of escalated immunosuppressive therapy was necessary in 2 of 9 surviving patients. Day 100 and 180 post infusion survival was 90% and 60%, respectively. Serum biomarker REG3α decreased, particularly in the high dose cohort, and with REG3α decrease correlated with clinical response.
Conclusions
Treatment of patients with de novo HR or SR aGVHD with low or high dose MSCTC-0010 was safe: the infusion was well-tolerated, and no TRAEs or ectopic tissue formation was observed. A clinical improvement was seen in about 70% patients, with 4 of 10 showing a complete response that may have been attributable to MSCTC-0010 infusions. These observations indicate safety of two different doses of MSCTC-0010, and suggest that the 10 × 106 cells/kg dose be tested in an expanded randomized, controlled Phase 2 trial.
Keywords: aGVHD, Wharton’s Jelly mesenchymal stromal cells, umbilical cord, steroid refractory, Ruxolitinib, allo-HCT
Graphical Abstract
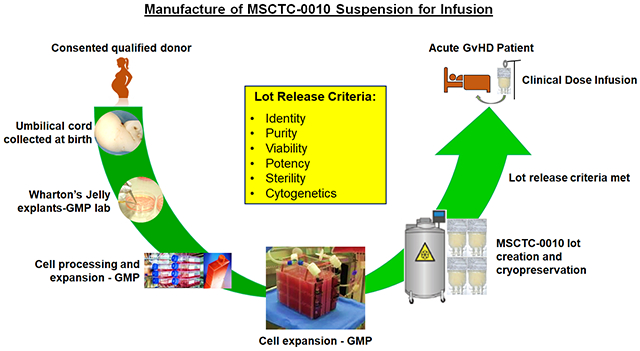
Introduction
Allo-HCT is commonly employed as potentially curative treatment strategy in the management of hematologic malignancies, bone marrow failure syndromes, and inborn errors of metabolism [1]. This complex procedure is frequently complicated by an immunologically mediated process, whereby donor T cells recognize recipient tissues as foreign and initiate a tissue destructive process called graft versus host disease (GVHD). GVHD represents a common cause of post-transplant morbidity and mortality [2–4].
Although GVHD presents as an acute (aGVHD) or chronic (cGVHD) clinical syndrome, with significant overlap, the most proximal cause of non-relapse morbidity and mortality post-Allo-HCT is represented by aGVHD, the extent of which is determined by a staging/grading system [5, 6, 7]. Patients with aGVHD Grades I-II experience 5-year leukemia-free survival of 44% to 51%; in contrast, survival decreases to 26% for patients with Grade III and 7% for Grade IV aGVHD [8].
The most effective approach to attenuate the risk and extent of aGVHD is to employ prophylactic strategies, the most common of which have included optimal major histocompatibility complex (MHC) Class I and II loci matching between donor and recipient; the pharmacologic blocking of T-cell antigen recognition and resultant proliferation, principally through the employment of calcineurin inhibitors; in-vivo or ex vivo T-cell depletion of the stem cell graft; or post-transplant cyclophosphamide [9–12]. Despite these measures, aGVHD remains a relatively common clinical challenge post Allo-HCT.
The standard initial treatment for aGVHD is glucocorticoid-based therapy (referred to as steroid therapy). Unfortunately, a significant percentage of patients will become resistant to steroid therapy and will subsequently be treated with second-line immunosuppressive agents [5, 6]. Steroid-refractory aGVHD (SR-aGVHD) portends a very poor prognosis, second-line agents frequently prove ineffective, and as a result, survival for these patients is < 10% at 5 years. Therefore, alternative therapies are needed to treat GVHD, particularly in the setting of steroid refractory disease. By using a combination of clinical and biological risk assessment tools, transplant physicians can predict which patients will require additional immunosuppression and have the highest risk for non-relapse mortality. The two validated systems that are most predictive of lack of response to steroids and GVHD lethality include a refined clinical risk score and serum or plasma biomarker-based risk score [13, 14]. These new approaches promise to inform a personalized, risk-adapted approach to therapy in the next generation of GVHD studies.
A promising treatment strategy for SR-aGVHD involves the infusion of third-party, HLA-unmatched, bone marrow derived mesenchymal stromal cells (BMMSC) [15–40]. The immunosuppressive properties of BMMSC suggest their potential use in a broad range of inflammatory immune-mediated conditions, such as GVHD. In general, mesenchymal stromal cells (MSCs) inhibit the activation and proliferation of T-cells that have been activated by a variety of stimuli. MSC immunomodulation is mediated through several mechanisms including the elaboration of immunosuppressive cytokines, down-regulation of inflammatory cytokine expression by activated T-cells, contact inhibition of dendritic cells and cytotoxic T-cells, and through the production of T-cell inhibitory extracellular vesicles [41–44].
Several studies indicate that adult-derived MSCs have reduced expansion potential or slower expansion compared to fetal tissue-derived MSCs, and adult MSCs may be less immunosuppressive than fetal or neonatal MSCs in certain applications [45–50]. In contrast, MSCs derived from discarded post-natal tissues might offer certain advantages over BMMSC for GVHD therapy. Our thesis is that umbilical cord derived MSCs may be an effective, safely and painlessly collected alternative source of MSCs for GVHD prevention or treatment.
Wharton’s Jelly is a primitive, loose connective tissue that is rich in hyaluronic acid, and that supports and cushions the umbilical vessels. Wharton’s Jelly contains an MSC population that is easily isolated following birth from the discarded umbilical cord. Wharton’s Jelly mesenchymal stromal cells (WJMSC) grow more quickly and produce more cells during in vitro expansion compared with BMMSC [51, 52], and they have immune-suppressive properties similar and possibly superior to adult-derived MSCs from bone marrow and adipose tissue [48, 51, 53–55]. WJMSC synthesize anti-inflammatory proteins and secrete biologically active extracellular vesicles [56–82].
In this report we describe the safety, impact on biological markers of aGVHD activity, and clinical outcomes of WJMSCs (MSCTC-0010), administered to ten patients in two-five patient cohorts (low and high dose of MSCs) with de novo HR or SR aGVHD post allogeneic stem cell transplantation.
Materials and Methods
Patients and Trial Design
This single-center, open-label, phase I study (clinicalrials.gov #NCT03158896) was designed to evaluate the safety of MSCTC-0010, (WJMSC, Suspension for Infusion), in the treatment of de novo HR or SR-aGVHD in adult patients. Patients were eligible if 18-75 years of age and had not received any other investigational agent used to treat aGVHD for 30 days prior to enrollment. The study was approved by the institutional review board and was conducted in accordance with the principles of the Declaration of Helsinki and International Conference on Harmonization Good Clinical Practice Guidelines [83]. All the patients provided written informed consent.
Patients were eligible for this trial if they failed to respond to systemic steroid treatment as first-line treatment for aGVHD, as defined by progression within 3 days or no improvement within 7 days of consecutive treatment with 1.6 mg/kg/d of methylprednisolone or equivalent. HR is defined by Minnesota criteria [13]. Patients were excluded if they had received any other investigational systemic therapy for the treatment of aGVHD for 30 days prior to enrolling.
Investigational Agent
MSCTC-0010, Suspension for Infusion, was manufactured by the Midwest Stem Cell Therapy Center (MSCTC) at the University of Kansas Medical Center under IND 017672 following explantation, expansion, formulation, and cryopreservation steps. The manufacturing process for rffollowing childbirth at the University of Kansas Hospital Labor and Delivery unit. Written informed consent, approved by the University of Kansas Medical Center Institutional Review Board (IRB), was obtained from mothers prior to umbilical cord collection. The umbilical cords (typically 15 to 20 cm long) were accepted from healthy full-term women (18-35 years old) who underwent elective cesarean section. In order to qualify as cord donors, mothers were tested and shown to be free of Human Immunodeficiency Virus (HIV) Types 1 & 2, Hepatitis A, B, and C, Treponema pallidum, Chlamydia trachomatis, Neisseria gonorrhea, and HTLV 1 and 2. WJMSCs were isolated, cultured and expanded under current Good Manufacturing Practice/Good Tissue Practice (cGMP/cGTP) standards. MSCs were harvested after passage 5.
MSCTC-0010 was cryopreserved in Plasmalyte-A supplemented with DMSO and human serum albumin. Before freezing, cells were characterized for MSC-expressed CD markers by flow cytometry using the BD Stemflow Human MSC analysis kit (BD Biosciences, San Diego, CA). Flow cytometry revealed that ≥95% of cells expressed the markers characteristic of MSCs (CD105, CD73, CD90, and CD44) while the expression of hematopoietic, macrophage, and B cell markers (CD45, CD34, CD11b, CD19 and HLA-DR) was 2% or less. We examined the extent of MSCTC-0010 to suppress proliferation of activated human peripheral blood mononuclear cells (PBMCs). PBMCs were labelled with 5 μM carboxyfluorescein succinimidyl ester (CFSE; eBioscience), stimulated with 1 μg/mL phytohemagglutinin (PHA; Sigma-Aldrich) and co cultured with MSCTC-0010 at a ratio of 1:10 for 3 days. The intensity of CFSE staining of PHA-induced proliferating PBMCs was evaluated by flow cytometry (Becton Dickenson LSR II flow cytometer). Data were analyzed using FACSDiva software. MSCTC-0010 cells suppressed mitogen-induced peripheral blood mononuclear cell activation and proliferation. Potency release criterion of each MSCTC-0010 lot was met as described in Table 1. MSCTC-0010 was determined to be sterile (USP<71> sterility test) and endotoxin free using the quality control test kit (Limulus amebocyte lysate method; Endosafe system). MSCTC-0010 was negative for mycoplasma (Mycoalert Mycoplasma detection kit), and had no chromosomal abnormalities (Cytogenetic analysis, University of Kansas Medical Center). MSCTC-0010 was thawed immediately on the day of administration and cell viability of ≥80 % using trypan blue was the final qualification for release (Table 1).
Table 1.
Quality Control of MSCTC-0010, Suspension for Infusion
| Analytical test | Method | Acceptance Criteria |
|---|---|---|
| Appearance | Off-white, opaque, homogeneous suspension following mixing | Visual, no clumps |
| Phenotype | Flow Cytometry | ≥ 80% CD73, CD90, CD105 ≤ 2% CD45, CD34, CD11b, CD19, HLA DR |
| Cell Viability | Flow Cytometry | ≥ 80% |
| Potency by Immunosuppression | Inhibition of PHA-induced proliferation of hPBMC by WJMSC | ≥ 20% |
| Endotoxin | Bacterial endotoxin USP <85> | < 12.5 EU/ml |
| Mycoplasma | Biochemical luminescence assay | Absence of mycoplasma |
| Sterility | Microbiological control of cellular product USP <71> | Absence of micro organisms |
| Chromosomal Stability | Karyotype | Absence of chromosomal alterations |
EU = endotoxin units, hPBMC= human peripheral blood mononuclear cells.
The first cohort of 5 patients received an intravenous dose of 2×106 viable MSCs/kg on days 0 and 7 (low dose cohort) and the second cohort of 5 patients received a dose of 10×106 MSCs/kg on days 0 and 7 (high dose cohort, maximum dose of 1000×106 cells per administration). The first dose was given within 120 hours of diagnosis of either HR or SR aGVHD Grade Ic-IV. Before the infusion, steroids were given to prevent an infusion reaction. During and after the MSC infusions, the patients maintained their baseline established therapy with systemic steroid therapy based on institutional algorithm and their GVHD prophylactic agent. In the event of disease progression, during the initial 7 days, additional medications for the treatment of aGVHD were allowed. In this study, 10 umbilical cord donors were used for 10 patients to manufacture MSCTC-0010, hence each patient received infusion of cells derived from one cord.
Assessment of Safety, Efficacy and Biomarker Response
GVHD assessments were performed weekly from enrollment through day 42 and on day 90. Survival was assessed at day 100 and 180. Severity of aGVHD for each patient was evaluated using the Consensus Conference on Acute GVHD Grading [84]. Presence or absence of aGVHD of the skin, liver, and gut was determined and graded according to the clinical assessment of each patient, as described in Study endpoints section. All untoward medical occurrences after the initial treatment were considered adverse events (AEs). Adverse events were graded based on Common Terminology Criteria for Adverse Events version 5.0 (CTCAE v5.0). Treatment Related Adverse Event (TRAE) is defined as a Grade 3 or greater adverse event, which is (1) at least probably related or definitely related to the MSCTC-0010 infusions, and (2) does not return to baseline within 24 hours from the start of the event. Serious AEs were defined according to International Conference on Harmonization E6 standards [83]. Ectopic tissue formation was an AE of special interest and patients were assessed at a prespecified time point with computed tomography imaging. Vital signs, physical examination, and laboratory assessments were performed at screening, weekly through study day 42, and at study day 90 and 180. Biomarkers ST2 and REG3-α were analyzed in blood serum samples collected 1 day prior to the MSCTC-0010 (day -1) infusion and on day 14 post infusion. Serum ST2 and REG3α concentrations were determined by sandwich enzyme-linked immunoassay (ELISA) as previously described [86] by Viracor-IBT Laboratories, Inc.
Study Endpoints
The primary endpoint was the proportion of participants reaching day 42 without a TRAE after the first infusion of MSCTC-0010. The study mandated a hold in enrollment pending a Data Safety Monitoring Committee determination if any participant experienced a TRAE or the threshold of participants in total experiencing a TRAE was reached, following treatment of the first cohort. There were additional stopping parameters for ectopic tissue formation and non-response to therapy. Secondary endpoints were evaluated based on the proportion of participants who achieved a complete response (CR) or improvement of aGVHD in 1 or more involved organs by study days 28 and 42, or required an escalation of immunosuppressive therapy within 90 days of the first dose of MSCTC-0010.
Response Criteria:
CR: resolution of aGVHD in all involved organs
PR: decrease by at least 1 GVHD stage in any 1 organ system without any worsening in any other organ system
Durable response: a response (e.g., improvement in GVHD grade) lasting for at least 28 days
Mixed response: improvement by at least 1 stage in at least 1 organ with worsening by at least 1 stage in at least 1 other organ
No response: stable or worsening disease
Stable disease: the absence of any clinically significant differences (Improvement or worsening) sufficient to meet minimal criteria for improvement or deterioration in any evaluable organ
Worsening: deterioration in at least 1 organ system by 1 stage or more with no improvements in any other organ
Flare: recurrence of aGVHD after a CR
Minnesota risk scoring defines HR as either baseline stage 4 skin, stage 3 to 4 lower GI, stage 3 to 4 liver, or stage 3 to 4 skin + stage 3 to 4 liver or lower GI, with all other patients classified as standard risk [13, 85].
After the first 5 participants were enrolled in the low dose cohort, they were followed for a full 42-day safety monitoring period. Subsequently, enrollment to the second cohort commenced if no safety concerns were evident in the first cohort. The DSMB reviewed and approved the safety data obtained in low-dose cohort before moving to the high dose cohort.
Statistical analysis:
Descriptive statistics summarize the patient population. Kaplan Meier survival curve was used to depict overall survival. The biomarker concentrations were analyzed in SigmaPlot14 (build 14.0.3.192) and expressed as box and whisker plots. Boxes contain the first and third quartiles and the median. Whiskers represent the 10th and 90th percentiles. To determine whether MSCs affected biomarker concentrations, paired t-test was used when the normality assumptions were met or Wilcoxon test was used when normality assumption was violated. The aGVHD score was calculated using a validated algorithm [14]. Significance was defined as p < 0.05 in two tailed testing.
Results
Patient characteristics:
Twelve patients were screened and ten patients were enrolled in the study between August 2018 and September 2019. All 10 patients completed the study and were included in this analysis. The median age of patients was 57.5 years (range of 35 – 73 yrs) and 7 were male. Five patients had high risk acute myelogenous leukemia (AML), 2 myelodysplastic syndrome (MDS), 2 myelofibrosis, and 1 had T-cell non-Hodgkin’s lymphoma (T-NHL). Five patients were transplanted with matched unrelated hematopoietic stem cell donors, 3 with matched related hematopoietic stem cell donors and 1 each with a haploidentical and an umbilical cord blood donor. Six received a myeloablative conditioning regimen and the remainder had a reduced intensity conditioning regimen. Five of the 8 patients who received hematopoietic stem cell grafts from matched unrelated or related donors received bone marrow grafts (Table 2).
Table 2.
Patient Demographics, aGvHD grade and response
| Disease | Age | Cohort | Graft source | Donor | Conditioning | aGVHD grade Dx | Rx Baseline | aGVHD at d0 | aGVHD d28 | D28 response | d90 PDN | Additional agent d90 | Survival d100 | Survival d180 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MDS Complex | 73 | 1 | BM | MUD | BuFlu | IIB GI d51 | PDN 2/kg, Tac B&B | IIB SR | 1B | Y | 70mg | none | Y | Y |
| MDS Complex | 63 | 1 | PBSC | MUD | BuCy | IIB GI d145 | PDN 2/kg, Tac B&B | IIIB HR | IIB | Y | 40mg | Rux | Y | N |
| PV/Myelofibrosis | 60 | 1 | PBSC | MRD | BuFlu | IIC GI, skin, liver d268 | PDN | IIC HR | 0 | Y | 15mg | none | Y | Y |
| AML | 48 | 1 | BM | MUD | BuCy | 1B SR aGvHD d53 | PDN 2mg/kg, ECP TacRux | 1B SR | 0 | Y | 0 | Rux | Y | Y |
| T-NHL | 65 | 1 | PBSC | HAPLO | FluCyTBI | IIC 2 weeks post Nivolumab | PDN 2mg/kg, Rux | IIC SR | IA | Y | 10mg | none | Y | Y |
| AML | 42 | 2 | PBSC | MUD | BuCy | IIB skin, GI d31 | MPD 1.6/kg, Tac B&B | IIB HR | 0 | Y | 0 | none | Y | Y |
| AML | 57 | 2 | BM | MRD | BuCy | IIB d30 | PDN 2/kg, Tac Rux | IIC SR | 0 | Y | 10mg | none | Y | Y |
| AML | 58 | 2 | BM | MRD | BuCy | IIIC d346 | MPD 1.6/kg, Tac B&B | IIB HR | IIB | N | 90mg | Rux | Y | N |
| AML | 35 | 2 | UCB | UCB | FluCyThio | IIB d20 | CSAMPD 1.6mg/kg | IIB HR | IIB | N | 45mg | none | Y | N |
| Myelofibrosis | 51 | 2 | BM | MUD | BuFluATG | IIIC d97 | MPD 1.6mg/kg, Tac B&B | IIIC SR | IV | N | NA | n/a | N | N |
AML = acute myeloid leukemia, MDS = Myelodysplastic syndromes, PV = polycythemia vera, T-NHL = T-cell non-Hodgkin lymphoma, MPD = methylprednisolone, PDN=prednisone, Rux= Ruxolitinib,Tac=tacrolimus, B&B= budesonide and beclomethasone, ECP=extracorporeal photopheresis, Bu=busulfan, Cy=Cytoxan, D=Day, Flu=fludarabine, Thio=thiotepa, SR= steroid refractory, HR=high risk, BM=bone marrow, PBSC= Peripheral blood stem cell, UCB=Umbilical Cord Blood, MUD= Matched Unrelated Donor, MRD= Matched Related Donor
Feasibility and Safety:
The enrolled patients received the first dose of MSCTC-0010 within 120 hours from the diagnosis of de novo HR or SR aGVHD as defined by the protocol. Furthermore, the second dose of MSCTC-0010 was administered one week after infusion of first dose. No patient experienced a TRAE throughout the follow up period. All patients experienced at least 1 adverse event (AE) and 7 patients experienced at least 1 grade 3 or 4 AE. The MSCTC-0010 infusion was tolerated with no associated acute toxicities. There were no AEs that were at least probably related or definitely related to the MSCTC-0010 infusions (Table 3). No patient had evidence of ectopic tissue formation based on CT imaging of the chest, abdomen and pelvis on day 90.
Table 3.
Adverse Events
| Adverse Events | Grade 1 | Grade 2 | Grade 3 | Grade 4 |
|---|---|---|---|---|
| Cardiac Disorder | 1 | 0 | 0 | 0 |
| Gastrointestinal Disorders | 6 | 2 | 1 | 0 |
| General Disorders | 6 | 4 | 1 | 0 |
| Genitourinary Disorders | 0 | 2 | 0 | 0 |
| Infections and Infestations | 1 | 2 | 4 | 1 |
| Investigations | 0 | 2 | 0 | 0 |
| Mental and Behavioral Disorders | 4 | 0 | 0 | 0 |
| Metabolism and Nutrition Disorders | 4 | 6 | 1 | 1 |
| Musculosketal and Connective Tissue Disorders | 0 | 4 | 0 | 0 |
| Nervous System Disorders | 3 | 0 | 1 | 0 |
| Renal and Urinary Disorders | 0 | 1 | 0 | 0 |
| Respiratory, Thoracic and Mediastinal Disorders | 1 | 0 | 0 | 1 |
| Skin and Subcutaneous Tissue Disorders | 6 | 1 | 1 | 0 |
Adverse events were unrelated to the cell therapy.
GVHD:
Five patients had SR-aGVHD (3 in low dose cohort and 2 in high dose cohort) and 5 had HR aGVHD (2 in low dose cohort and 3 in high dose cohort). The median time post allo-HCT for the development of aGVHD was day 75 (range 20 – 340 days). Acute GVHD grade on day 0 of the MSCTC-0010 infusion was IIB or higher in 9 patients and IB in a patient with steroid refractory disease. All patients received a minimum of 1.6 mg/kg/day methylprednisolone or equivalent at onset of their aGVHD. Additionally, 7 patients were receiving prophylaxis with tacrolimus, and 1 cyclosporine. Five patients with gastrointestinal (GI) involvement were also receiving oral beclomethasone and budesonide and 3 patients were receiving Ruxolitinib. Topical steroids were used in 5 patients with skin involvement.
Ruxolitinib and extracorporeal photopheresis were added in 3 of the patients with stable or progressive aGVHD that started 10 days to 3 weeks after WJMSC infusion. By day 28, 4 patients were in CR, 3 PR, 2 stable and 1 had progression of aGVHD. By day 90, 9 patients were alive, one had relapsed leukemia, 2 patients had tapered off prednisone, 3 were receiving ≤15 mg daily and tapering, 3 were receiving 70 mg or less and tapering and one with refractory aGVHD was receiving 90 mg (Figure 1A). Three of 9 surviving patients required an additional agent by day 90 (Ruxolitinib).
Figure 1.
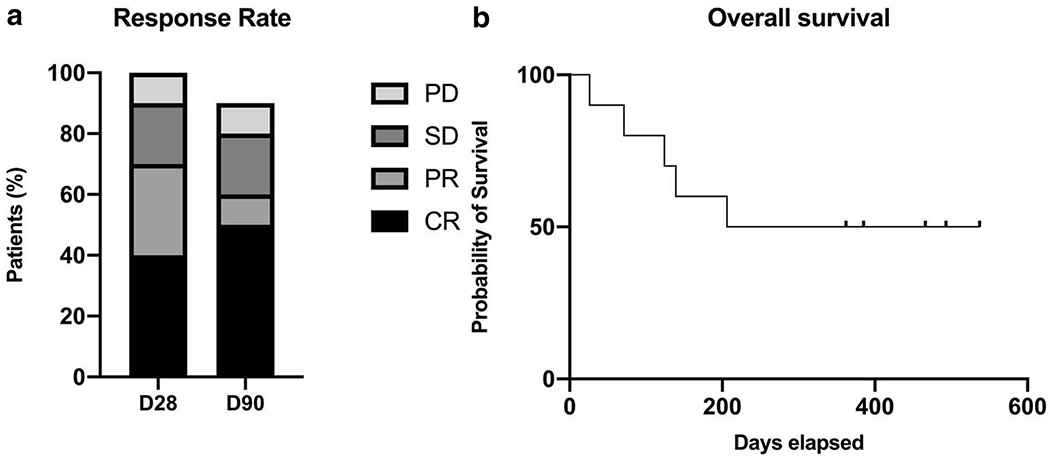
Response rate and overall survival. A. Response rate at day 28 and day 90. B. Overall survival.
CR=complete remission, D28= Day 28, D90=Day 90, PR=partial remission, PD= Progressive disease, and SD= stable disease.
Disease relapse and infections:
Two patients had disease relapse including one patient with relapsed T-NHL who attained a sustained complete remission after treatment and remains alive. Six patients developed infections during the 90 day follow up after MSCTC-0010 infusion. Two patients had CMV reactivation, two developed pneumonia, one patient developed bacteremia and one developed Clostridium difficile, as well as BK and HHV6 viremia.
Overall Survival:
The median overall survival was 372 days (Figure 1B). By day 180, 4 patients had expired, one from primary malignancy relapse and 3 from progression of aGVHD. At last follow-up in April 2020, 5 of the 10 patients were alive, two with chronic GVHD on tapering doses of steroids.
Biomarkers:
As shown in figure 2, samples were collected 1 day prior to MSCTC-0010 infusion (day -1) and on day 14 after the first dose infusion. ELISA results were then used to calculate the GVHD algorithm score. Six of the 10 patients on the study had an elevated GVHD algorithm score prior to infusion. Four of 5 patients in the high dose MSCTC-0010 cohort and 3 of the patients in the low dose cohort had a reduction in their GVHD algorithm scores following treatment at day 14. Subjects from dose cohort 2 appeared to have had a steeper decline in serum REG3α concentrations between day 0 and day 14 although this did not meet statistical significance (Figure 2).
Figure 2.
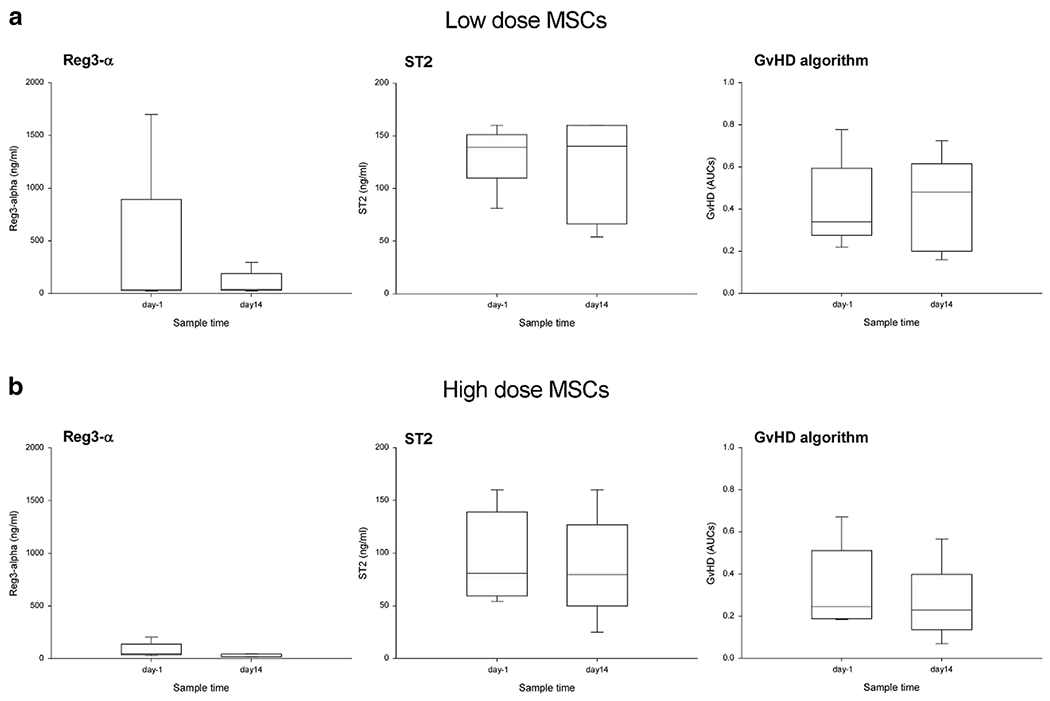
Reg3-α and ST2 concentrations and GvHD predictive algorithm on one day prior to infusion (day -1) and at day 14 (day 14) post infusion of first dose. Panel A shows results from low dose MSCT-0010 cohort. Biomarkers Reg3-α (left) and ST2 (middle) are not significantly different after receiving MSCT-0010 treatment. There was a trend for Reg3-α to decrease after MSC infusions (compare day-1 and day 14). The GvHD algorithm AUCs were not significantly different after MSC infusions. There was a trend for GVHD score to increase. Panel B shows patients in the high dose MSCT-0010 cohort. Biomarkers Reg3-α (left) and ST2 are not significantly different after MSC infusions. There was a stronger trend for Reg3-α to decrease after high dose MSC infusions than low dose MSC infusion (compare effect size of Reg3-α between panels A and B). The GvHD algorithm AUCs were not significantly different between the two samples. There was a trend for GVHD score to decrease in high dose cohort.
Discussion
While bone marrow derived MSCs have been GMP manufactured for many years and have passed through the customary three phases of clinical testing as a salvage treatment for GVHD, the present study represents the first Phase 1 trial of WJMSCs for GVHD within the United States. Our principle finding was that WJMSCs appear to be safe for SR and HR aGVHD patients when given at either a “low-dose” of 2×106 or a “high-dose” of 10×106 cells/kg delivered intravenously twice, one week apart.
“Safety” was evaluated here in three ways and found to be safe. First, an acute response to MSC infusion or “infusional toxicity” was evaluated both during intravenous delivery of either a low or high dose. In the immediate period after MSC infusion, no AEs were noted. The lack of infusion-linked AEs is important because previous reports raised the concern that MSC infusion might be linked with emboli formation or respiratory distress following their becoming lodged in the first capillary bed they encounter. Second, study patients lacked TRAEs during the 42-day observation period, a primary outcome measure. While AEs were recorded for all study patients, and seven of the ten patients recorded a grade 3 or higher AEs, none of the AEs were attributed to experimental therapy. In this regard, it should be noted that SR and HR GVHD patients are complicated cases to manage, and require balancing immune suppression to mitigate GHVD damage against the development of infection or infestation. Thus, that AEs in five of these seven patients with grade 3 or higher might be attributed to immunosuppression. This observation should be marked since one might pose that the higher rate of infections or infestation might be a secondary consequence of MSC-mediate immune suppression. Others have suggested that MSC treatment for GVHD might introduce infection risk for or might reduce the “graft verse tumor” effect gained by allo-HSCT. We did not observe a higher rate of infection or relapse in the SR and HR aGVHD patients, here. This concern represents a feature that needs further investigation using larger cohorts and using a well-designed, randomized controlled trial. Third, theoretically, the administration of third-party, HLA-disparate, WJMSCs to an immunosuppressed patient could lead to their engraftment and subsequently the formation of ectopic tissue. Consistent with other MSC studies of management of SR-aGVHD, none of the patients here developed ectopic tissue formation at day 90 post first infusion in either of the 2 cohorts [23, 38, 39]. Taken together this phase I safety study indicates that WJMSCs are safe for treating SR and HR aGVHD patients up to a dose of 10×106 cells per kg given twice, one week apart.
Acute GVHD represents a common cause of morbidity and mortality in patients undergoing a potentially curative allo-HCT transplant for life threatening hematologic disorders. Despite strategies to prevent aGVHD, a significant percentage of patients, dependent on transplant specific variables, will develop aGVHD, and 30-50% will prove to be SR-aGVHD [1, 5, 87, 88]. HR aGVHD was included in this study because of the previously demonstrated significantly decreased likelihood of this group responding to steroid therapy at day 28 and the increased overall and transplant related mortality of HR aGVHD patients compared to standard risk patients [13]. The addition of secondary immunosuppressive agents to steroids in the treatment of aGVHD have failed to demonstrate a beneficial effect [89]. While a Phase I trial is designed to evaluate safety and dose-limiting toxicity, it may also provide an estimate or a glimpse of the efficacy to treat SR or HR GVHD. On the other, hand Phase 1 studies customary enroll a small numbers of patients to mitigate risks. For example, here, five patients were evaluated per dose cohort, and all patients were treated. Thus, size and design reduce the ability of our study to estimate MSC clinical effect or efficacy.
Despite these limitations, we endeavored to estimate efficacy of MSC-treatment by observing GVHD clinical score, discussed in the paragraph, and GVHD biomarkers, discussed below. Here, positive clinical responses were observed in HR or SR-aGVHD patients. By study day 28, 70% of patients experienced an ORR; 40% had a CR and 30% a PR. By study day 90, the addition of escalated immunosuppressive therapy was necessary in 3 patients. Day 100 and 180 post infusion survival was 90% and 60%, respectively and consistent with the survival demonstrated in similar populations of patients treated with secondary investigational agents [38–40, 90]. Whether the WJMSC infusions contributed to these outcomes cannot be determined definitively here and will require well-designed and well-powered follow-on Phase 2 studies. Importantly, 3 patients were treated concomitantly with Ruxolitinib and these patients were among those achieving an ORR by day 28.
Here, positive clinical response was observed after WJMSC infusions. To place our observations into a context, the clinical effects of Ruxolitinib, the only Food and Drug Administration-approved therapy for SR-aGVHD, were estimated in a recent study [90]. Adding Ruxolitinib led to, at day 28, an overall response rate (ORR) of 62% and 34% achieved complete responses (CR), compared to 39% and 19% in the control arm (investigator’s choice of therapy). Durable ORR at day 56 was 40% for the group receiving Ruxolitinib. Thus, the estimated clinical response and effect size observed in this small SR or HR aGVHD patients was more similar to Ruxolitinib than the control arm.
Clinical studies utilizing BMMSC have demonstrated variable outcomes and differences in tissue source and manufacturing, cell dose and timing of administration makes comparisons across studies difficult. Recently, a multicenter, randomized, double-blind, placebo-controlled study of Remestemcel-L for pediatric and adult patients with SR aGVHD demonstrated safety but no significant benefit over placebo when added to second line therapy. In post hoc analyses, specific patient subgroups appeared to benefit from Remestemcel-L treatment included pediatric patients, patients with any liver involvement, and patients with high-risk aGVHD [40]. In contrast, recent results from an open-label Phase 3, multicenter trial in 54 children with SR-aGVHD was reported using Remestemcel-L. The pediatric population was dosed at 2×106 cells/kg twice weekly for 4 weeks. The primary endpoint of day 28 ORR was achieved in 70%, which was sustained through day 100, including an increase in complete response from 29.6% at day 28 to 44.4% at day 100. Overall survival was 74% at day 100 [24]. These results were further supported by results of an Expanded Access Program in 241 children where Remestemcel-L was used as salvage therapy after failure of steroids and other agents [39]. Important questions remain regarding the optimization of MSC treatment of aGVHD, including the effects of manufacturing, cell dose, the frequency of administration, and the optimal source of MSCs.
There is a great deal of interest in biomarkers that predict GVHD development, disease progression or response to therapeutic intervention (see review Paczesny S, Blood 131(20): 2193-204, 2018). Furthermore, changes in GVHD biomarkers may provide indication of the mechanism of action used by MSCs to treat GVHD. For that reason, two GVHD biomarkers were evaluated here before MSC treatment and on day 14, after two MSC infusions. Major-Monfried et al. reported a validated algorithm of 2 serum biomarkers, ST2 and REG3α, which separated SR-aGVHD into 2 groups with dramatically different non-relapse mortality (NRM) and overall survival. High biomarker probability, resistance to steroids and GVHD severity were all significant predictors of NRM in multivariate analysis [99]. For that reason, we evaluate ST2 and REG3-α levels here, and interestingly, as shown in figure 2, REG3-α tended to decrease from day-1 to day 14 after MSC infusion, particularly in the high MSC dose cohort. The decrease in REG3-α levels correlated with clinical response (data not shown), but the decrease in REG3-α did not reach significance here. In planning follow-on studies, REG3-α and ST2 concentrations should be evaluated to determine if they predict GVHD progression or response to MSC therapy. It is anticipated that, as the field matures, biomarkers monitoring will enable the development of personalize GVHD treatment plans and mechanistic selection of targeted interventions.
WJMSC represent an alternative MSC source with certain advantages that bear consideration as a cell for therapeutic application. These cells have been demonstrated, both in our laboratory and others, to proliferate robustly and consistently ex-vivo; maintain cytogenetic stability and their broad immunosuppressive properties through multiple passages; are minimally immunogenic; suppress proliferation of activated T-cells, increase production of regulatory T-cells, inhibit dendritic cell trafficking to lymph nodes and antigen presentation; do not stimulate B cells; shift the immune response towards tolerance or anergy; and produce exosomes with T-cell inhibitory properties [48, 51–55, 71–80]. Because of these properties, WJMSCs bear consideration as an alternative to bone marrow MSCs for therapy. Additional considerations in favor of WJMSCs is the ability for advanced manufacturing and qualification of WJMSCs to enable rapid clinical deployment.
Summary
Based on preclinical research using WJMSCs, our group undertook a ten patient Phase 1, two dose cohort study of MSCTC-0010 in the treatment of either HR or SR-aGVHD. The primary endpoint of this study was to determine the safety MSCTC-0010 as determined by lack of acute infusional toxicity, lack of TRAEs at day 42 or ectopic tissue formation at day 90 post first infusion in either of the 2 cohorts. Using these measures, WJMSCs were deemed “safe”. Given the immunosuppressive properties of WJMSCs, concerns regarding the potentiation of infection and relapse are appropriate. Based upon this small study, it was not possible to definitively conclude that WJMSCs lack adverse outcomes, but no treatment-related AEs were observed here. A secondary endpoint was to gain an estimate of clinical effect and effect size. Overall, we observed positive clinical response and clinical effects that are more comparable in response rate and clinical effect size to Ruxolitinib or Remestemcel-L than standard of care. That said, a phase 2 clinical trial that includes double masked, randomized, placebo controlled design including SR and HR-aGVHD population would be required to provide stronger evidence of WJMSCs’ clinical effects and adverse outcomes, if any. Due to the number of GVHD patients larger studies are more difficult to complete in a timely fashion, and a multi-center approach would be more fitting for follow-on studies and also provide stronger clinical evidence.
Conclusions
Ten HR or SR aGVHD patients were treated with one of two doses of MSCTC-0010 in a Phase I study. Overall we found that MSC treatment appears to be safe and well-tolerated. Our secondary outcome was to estimate clinical effect size to enable follow-on efficacy testing. From this data, it appears that MSCs treatment is a potentially effective, with an estimated effect size similar to Ruxolitinib or Remestemcel-L. Further studies of MSCTC-0010 are warranted in the treatment of aGVHD.
Acknowledgments
We acknowledge Mary Pilcher-Cook, a legislator in the Kansas state House and Senate, for her unwavering support for umbilical cord derived mesenchymal stromal cell research. The authors thank Carol Early for her extensive assistance and insightful suggestions, careful proofreading, editing, formatting, and organization of the manuscript. Funding: Research reported in this publication was supported by the National Cancer Institute Cancer Center Support Grant P30 CA168524. This work was supported by a CTSA grant from NCATS awarded to the University of Kansas for Frontiers: University of Kansas Clinical and Translational Science Institute (# UL1TR002366). The Midwest Stem Cell Therapy Center at University of Kansas and the Midwest Institute for Comparative Stem Cell Biotechnology are supported by the State of Kansas. The contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH or NCATS.
Footnotes
Conflict Of Interest
1. Rupal P. Soder- patent for GvHD applications of WJMSCs
2. Buddhadeb Dawn- patent for GvHD applications of WJMSCs
3. Mark L. Weiss- patent for GvHD applications of WJMSCs
4. Neil Dunavin- No conflicts to disclose
5. Scott Weir- No conflicts to disclose
6. James Mitchell- patent for GvHD applications of WJMSCs; employed and now consult for the Midwest Stem Cell Therapy Center during the development of MSCTC-0010
7. Meizhang Li- No conflicts to disclose
8. Leyla Shune- No conflicts to disclose
9. Anurag K. Singh- No conflicts to disclose
10. Siddhartha Ganguly- No conflicts to disclose
11. Marc Morrison- No conflicts to disclose
12. Haitham Abdelhakim- No conflicts to disclose
13. Andrew K. Godwin- Co-Founder of Sinochips Diagnostics
14. Sunil Abhyankar- Director of Midwest Stem Cell Therapy Center; patent for GvHD applications of WJMSCs
15. Joseph McGuirk- patent for GvHD application mu7 ns of WJMSCs
References
- 1.Gooley TA, et al. , Reduced mortality after allogeneic hematopoietic-cell transplantation. N Engl J Med, 2010. 363(22): p. 2091–101 DOI: 10.1056/NEJMoa1004383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bacigalupo A, Management of acute graft-versus-host disease. Br J Haematol, 2007. 137(2): p. 87–98 DOI: 10.1111/j.1365-2141.2007.06533.x. [DOI] [PubMed] [Google Scholar]
- 3.Lee SJ, New approaches for preventing and treating chronic graft-versus-host disease. Blood, 2005. 105(11): p. 4200–6 DOI: 10.1182/blood-2004-10-4023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wagner JE, et al. , Effect of graft-versus-host disease prophylaxis on 3-year disease-free survival in recipients of unrelated donor bone marrow (T-cell Depletion Trial): a multi-centre, randomised phase II-III trial. Lancet, 2005. 366(9487): p. 733–41 DOI: 10.1016/s0140-6736(05)66996-6. [DOI] [PubMed] [Google Scholar]
- 5.Pidala J and Anasetti C, Glucocorticoid-refractory acute graft-versus-host disease. Biol Blood Marrow Transplant, 2010. 16(11): p. 1504–18 DOI: 10.1016/j.bbmt.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 6.Wolff D, et al. , Consensus conference on clinical practice in chronic graft-versus-host disease (GVHD): first-line and topical treatment of chronic GVHD. Biol Blood Marrow Transplant, 2010. 16(12): p. 1611–28 DOI: 10.1016/j.bbmt.2010.06.015. [DOI] [PubMed] [Google Scholar]
- 7.Glucksberg H, et al. , Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation, 1974. 18(4): p. 295–304 DOI: 10.1097/00007890-197410000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Gratwohl A, et al. , Acute graft-versus-host disease: grade and outcome in patients with chronic myelogenous leukemia. Working Party Chronic Leukemia of the European Group for Blood and Marrow Transplantation. Blood, 1995. 86(2): p. 813–8. [PubMed] [Google Scholar]
- 9.Choi SW and Reddy P, Current and emerging strategies for the prevention of graft-versus-host disease. Nat Rev Clin Oncol, 2014. 11(9): p. 536–47 DOI: 10.1038/nrclinonc.2014.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bryant AR and Perales M-A, Advances in ex vivo T cell depletion – where do we stand? ADVANCES IN CELL AND GENE THERAPY, 2019. 2(1): p. e29 DOI: 10.1002/acg2.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qin B. z., et al. , Role of ATG in patients with hematologic diseases undergoing umbilical cord blood transplantation: A systematic review and meta-analysis. Clinical Transplantation. n/a(n/a): p. e13876 DOI: 10.1111/ctr.13876. [DOI] [PubMed] [Google Scholar]
- 12.Nunes NS and Kanakry CG, Mechanisms of Graft-versus-Host Disease Prevention by Post-transplantation Cyclophosphamide: An Evolving Understanding. Front Immunol, 2019. 10: p. 2668 DOI: 10.3389/fimmu.2019.02668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.MacMillan ML, et al. , A refined risk score for acute graft-versus-host disease that predicts response to initial therapy, survival, and transplant-related mortality. Biol Blood Marrow Transplant, 2015. 21(4): p. 761–7 DOI: 10.1016/j.bbmt.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levine JE, et al. , A prognostic score for acute graft-versus-host disease based on biomarkers: a multicentre study. Lancet Haematol, 2015. 2(1): p. e21–9 DOI: 10.1016/s2352-3026(14)00035-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Le Blanc K, et al. , Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet, 2004. 363(9419): p. 1439–41 DOI: 10.1016/s0140-6736(04)16104-7. [DOI] [PubMed] [Google Scholar]
- 16.Ringden O, et al. , Mesenchymal stem cells for treatment of therapy-resistant graft-versus-host disease. Transplantation, 2006. 81(10): p. 1390–7 DOI: 10.1097/01.tp.0000214462.63943.14. [DOI] [PubMed] [Google Scholar]
- 17.Arima N, et al. , Single intra-arterial injection of mesenchymal stromal cells for treatment of steroid-refractory acute graft-versus-host disease: a pilot study. Cytotherapy, 2010. 12(2): p. 265–8 DOI: 10.3109/14653240903390795. [DOI] [PubMed] [Google Scholar]
- 18.Baron F, et al. , Cotransplantation of mesenchymal stem cells might prevent death from graft-versus-host disease (GVHD) without abrogating graft-versus-tumor effects after HLA-mismatched allogeneic transplantation following nonmyeloablative conditioning. Biol Blood Marrow Transplant, 2010. 16(6): p. 838–47 DOI: 10.1016/j.bbmt.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 19.Fang B, et al. , Treatment of severe therapy-resistant acute graft-versus-host disease with human adipose tissue-derived mesenchymal stem cells. Bone Marrow Transplant, 2006. 38(5): p. 389–90 DOI: 10.1038/sj.bmt.1705457. [DOI] [PubMed] [Google Scholar]
- 20.Fang B, et al. , Using human adipose tissue-derived mesenchymal stem cells as salvage therapy for hepatic graft-versus-host disease resembling acute hepatitis. Transplant Proc, 2007. 39(5): p. 1710–3 DOI: 10.1016/j.transproceed.2007.02.091. [DOI] [PubMed] [Google Scholar]
- 21.Fang B, et al. , Human adipose tissue-derived mesenchymal stromal cells as salvage therapy for treatment of severe refractory acute graft-vs.-host disease in two children. Pediatr Transplant, 2007. 11(7): p. 814–7 DOI: 10.1111/j.1399-3046.2007.00780.x. [DOI] [PubMed] [Google Scholar]
- 22.Fang B, et al. , Cotransplantation of haploidentical mesenchymal stem cells to enhance engraftment of hematopoietic stem cells and to reduce the risk of graft failure in two children with severe aplastic anemia. Pediatr Transplant, 2009. 13(4): p. 499–502 DOI: 10.1111/j.1399-3046.2008.01002.x. [DOI] [PubMed] [Google Scholar]
- 23.Kebriaei P, et al. , Adult human mesenchymal stem cells added to corticosteroid therapy for the treatment of acute graft-versus-host disease. Biol Blood Marrow Transplant, 2009. 15(7): p. 804–11 DOI: 10.1016/j.bbmt.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 24.Kurtzberg J, et al. , Allogeneic human mesenchymal stem cell therapy (remestemcel-L, Prochymal) as a rescue agent for severe refractory acute graft-versus-host disease in pediatric patients. Biol Blood Marrow Transplant, 2014. 20(2): p. 229–35 DOI: 10.1016/j.bbmt.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 25.Le Blanc K, et al. , Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet, 2008. 371(9624): p. 1579–86 DOI: 10.1016/s0140-6736(08)60690-x. [DOI] [PubMed] [Google Scholar]
- 26.Lucchini G, et al. , Platelet-lysate-expanded mesenchymal stromal cells as a salvage therapy for severe resistant graft-versus-host disease in a pediatric population. Biol Blood Marrow Transplant, 2010. 16(9): p. 1293–301 DOI: 10.1016/j.bbmt.2010.03.017. [DOI] [PubMed] [Google Scholar]
- 27.Martin PJ, et al. , Prochymal Improves Response Rates In Patients With Steroid-Refractory Acute Graft Versus Host Disease (SR-GVHD) Involving The Liver And Gut: Results Of A Randomized, Placebo-Controlled, Multicenter Phase III Trial In GVHD. Biology of Blood and Marrow Transplantation, 2010. 16(2): p. S169–S170 DOI: 10.1016/j.bbmt.2009.12.057. [DOI] [Google Scholar]
- 28.Muller I, et al. , Application of multipotent mesenchymal stromal cells in pediatric patients following allogeneic stem cell transplantation. Blood Cells Mol Dis, 2008. 40(1): p. 25–32 DOI: 10.1016/j.bcmd.2007.06.021. [DOI] [PubMed] [Google Scholar]
- 29.von Bonin M, et al. , Treatment of refractory acute GVHD with third-party MSC expanded in platelet lysate-containing medium. Bone Marrow Transplant, 2009. 43(3): p. 245–51 DOI: 10.1038/bmt.2008.316. [DOI] [PubMed] [Google Scholar]
- 30.Weng JY, et al. , Mesenchymal stem cell as salvage treatment for refractory chronic GVHD. Bone Marrow Transplant, 2010. 45(12): p. 1732–40 DOI: 10.1038/bmt.2010.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou H, et al. , Efficacy of bone marrow-derived mesenchymal stem cells in the treatment of sclerodermatous chronic graft-versus-host disease: clinical report. Biol Blood Marrow Transplant, 2010. 16(3): p. 403–12 DOI: 10.1016/j.bbmt.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 32.Ball LM, et al. , Multiple infusions of mesenchymal stromal cells induce sustained remission in children with steroid-refractory, grade III-IV acute graft-versus-host disease. Br J Haematol, 2013. 163(4): p. 501–9 DOI: 10.1111/bjh.12545. [DOI] [PubMed] [Google Scholar]
- 33.Introna M, et al. , Treatment of graft versus host disease with mesenchymal stromal cells: a phase I study on 40 adult and pediatric patients. Biol Blood Marrow Transplant, 2014. 20(3): p. 375–81 DOI: 10.1016/j.bbmt.2013.11.033. [DOI] [PubMed] [Google Scholar]
- 34.Resnick IB, et al. , Treatment of severe steroid resistant acute GVHD with mesenchymal stromal cells (MSC). Am J Blood Res, 2013. 3(3): p. 225–38. [PMC free article] [PubMed] [Google Scholar]
- 35.Ringden O, et al. , Fetal membrane cells for treatment of steroid-refractory acute graft-versus-host disease. Stem Cells, 2013. 31(3): p. 592–601 DOI: 10.1002/stem.1314. [DOI] [PubMed] [Google Scholar]
- 36.Sanchez-Guijo F, et al. , Sequential third-party mesenchymal stromal cell therapy for refractory acute graft-versus-host disease. Biol Blood Marrow Transplant, 2014. 20(10): p. 1580–5 DOI: 10.1016/j.bbmt.2014.06.015. [DOI] [PubMed] [Google Scholar]
- 37.Wu KH, et al. , Effective treatment of severe steroid-resistant acute graft-versus-host disease with umbilical cord-derived mesenchymal stem cells. Transplantation, 2011. 91(12): p. 1412–6 DOI: 10.1097/TP.0b013e31821aba18. [DOI] [PubMed] [Google Scholar]
- 38.Kurtzberg J, et al. , A Phase 3, Single-Arm, Prospective Study of Remestemcel-L, Ex Vivo Culture-Expanded Adult Human Mesenchymal Stromal Cells for the Treatment of Pediatric Patients Who Failed to Respond to Steroid Treatment for Acute Graft-versus-Host Disease. Biol Blood Marrow Transplant, 2020. DOI: 10.1016/j.bbmt.2020.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kurtzberg J, et al. , Study 275: Updated Expanded Access Program for Remestemcel-L in Steroid-Refractory Acute Graft-versus-Host Disease in Children. Biol Blood Marrow Transplant, 2020. DOI: 10.1016/j.bbmt.2020.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kebriaei P, et al. , A Phase 3 Randomized Study of Remestemcel-L versus Placebo Added to Second-Line Therapy in Patients with Steroid-Refractory Acute Graft-versus-Host Disease. Biol Blood Marrow Transplant, 2019. DOI: 10.1016/j.bbmt.2019.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tse WT, et al. , Suppression of allogeneic T-cell proliferation by human marrow stromal cells: implications in transplantation. Transplantation, 2003. 75(3): p. 389–97 DOI: 10.1097/01.Tp.0000045055.63901.A9. [DOI] [PubMed] [Google Scholar]
- 42.Di Nicola M, et al. , Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood, 2002. 99(10): p. 3838–43 DOI: 10.1182/blood.v99.10.3838. [DOI] [PubMed] [Google Scholar]
- 43.Aggarwal S and Pittenger MF, Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood, 2005. 105(4): p. 1815–22 DOI: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- 44.Dander E, et al. , Understanding the Immunomodulatory Effect of Mesenchymal Stem Cell Infused In Transplanted Patients with Steroid-Refractory GvHD. Blood, 2010. 116(21): p. 2306–2306 DOI: 10.1182/blood.V116.21.2306.2306. [DOI] [Google Scholar]
- 45.Baksh D, Yao R, and Tuan RS, Comparison of proliferative and multilineage differentiation potential of human mesenchymal stem cells derived from umbilical cord and bone marrow. Stem Cells, 2007. 25(6): p. 1384–92 DOI: 10.1634/stemcells.2006-0709. [DOI] [PubMed] [Google Scholar]
- 46.Lund RD, et al. , Cells isolated from umbilical cord tissue rescue photoreceptors and visual functions in a rodent model of retinal disease. Stem Cells, 2007. 25(3): p. 602–11 DOI: 10.1634/stemcells.2006-0308. [DOI] [PubMed] [Google Scholar]
- 47.Wang L, et al. , A comparison of human bone marrow-derived mesenchymal stem cells and human umbilical cord-derived mesenchymal stromal cells for cartilage tissue engineering. Tissue Eng Part A, 2009. 15(8): p. 2259–66 DOI: 10.1089/ten.tea.2008.0393. [DOI] [PubMed] [Google Scholar]
- 48.Yoo KH, et al. , Comparison of immunomodulatory properties of mesenchymal stem cells derived from adult human tissues. Cell Immunol, 2009. 259(2): p. 150–6 DOI: 10.1016/j.cellimm.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 49.Zhang H, et al. , Increasing donor age adversely impacts beneficial effects of bone marrow but not smooth muscle myocardial cell therapy. Am J Physiol Heart Circ Physiol, 2005. 289(5): p. H2089–96 DOI: 10.1152/ajpheart.00019.2005. [DOI] [PubMed] [Google Scholar]
- 50.Zhang ZY, et al. , Superior osteogenic capacity for bone tissue engineering of fetal compared with perinatal and adult mesenchymal stem cells. Stem Cells, 2009. 27(1): p. 126–37 DOI: 10.1634/stemcells.2008-0456. [DOI] [PubMed] [Google Scholar]
- 51.Deuse T, et al. , Immunogenicity and immunomodulatory properties of umbilical cord lining mesenchymal stem cells. Cell Transplant, 2011. 20(5): p. 655–67 DOI: . [DOI] [PubMed] [Google Scholar]
- 52.Zeddou M, et al. , The umbilical cord matrix is a better source of mesenchymal stem cells (MSC) than the umbilical cord blood. Cell Biol Int, 2010. 34(7): p. 693–701 DOI: 10.1042/cbi20090414. [DOI] [PubMed] [Google Scholar]
- 53.Najar M, et al. , Adipose-tissue-derived and Wharton’s jelly-derived mesenchymal stromal cells suppress lymphocyte responses by secreting leukemia inhibitory factor. Tissue Eng Part A, 2010. 16(11): p. 3537–46 DOI: 10.1089/ten.TEA.2010.0159. [DOI] [PubMed] [Google Scholar]
- 54.Prasanna SJ, et al. , Pro-inflammatory cytokines, IFNgamma and TNFalpha, influence immune properties of human bone marrow and Wharton jelly mesenchymal stem cells differentially. PLoS One, 2010. 5(2): p. e9016 DOI: 10.1371/journal.pone.0009016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Weiss ML, et al. , Immune properties of human umbilical cord Wharton’s jelly-derived cells. Stem Cells, 2008. 26(11): p. 2865–74 DOI: 10.1634/stemcells.2007-1028. [DOI] [PubMed] [Google Scholar]
- 56.Li H, et al. , CCR7 guides migration of mesenchymal stem cell to secondary lymphoid organs: a novel approach to separate GvHD from GvL effect. Stem Cells, 2014. 32(7): p. 1890–903 DOI: 10.1002/stem.1656. [DOI] [PubMed] [Google Scholar]
- 57.Liu GY, et al. , Secreted galectin-3 as a possible biomarker for the immunomodulatory potential of human umbilical cord mesenchymal stromal cells. Cytotherapy, 2013. 15(10): p. 1208–17 DOI: 10.1016/j.jcyt.2013.05.011. [DOI] [PubMed] [Google Scholar]
- 58.Gieseke F, et al. , Human multipotent mesenchymal stromal cells use galectin-1 to inhibit immune effector cells. Blood, 2010. 116(19): p. 3770–9 DOI: 10.1182/blood-2010-02-270777. [DOI] [PubMed] [Google Scholar]
- 59.Sioud M, et al. , Evidence for the involvement of galectin-3 in mesenchymal stem cell suppression of allogeneic T-cell proliferation. Scand J Immunol, 2010. 71(4): p. 267–74 DOI: 10.1111/j.1365-3083.2010.02378.x. [DOI] [PubMed] [Google Scholar]
- 60.Ungerer C, et al. , Galectin-9 is a suppressor of T and B cells and predicts the immune modulatory potential of mesenchymal stromal cell preparations. Stem Cells Dev, 2014. 23(7): p. 755–66 DOI: 10.1089/scd.2013.0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bartosh TJ, et al. , Aggregation of human mesenchymal stromal cells (MSCs) into 3D spheroids enhances their antiinflammatory properties. Proc Natl Acad Sci U S A, 2010. 107(31): p. 13724–9 DOI: 10.1073/pnas.1008117107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Choi H, et al. , Anti-inflammatory protein TSG-6 secreted by activated MSCs attenuates zymosan-induced mouse peritonitis by decreasing TLR2/NF-kappaB signaling in resident macrophages. Blood, 2011. 118(2): p. 330–8 DOI: 10.1182/blood-2010-12-327353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kota DJ, et al. , TSG-6 produced by hMSCs delays the onset of autoimmune diabetes by suppressing Th1 development and enhancing tolerogenicity. Diabetes, 2013. 62(6): p. 2048–58 DOI: 10.2337/db12-0931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lee RH, et al. , Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell, 2009. 5(1): p. 54–63 DOI: 10.1016/j.stem.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Oh JY, et al. , Anti-inflammatory protein TSG-6 reduces inflammatory damage to the cornea following chemical and mechanical injury. Proc Natl Acad Sci U S A, 2010. 107(39): p. 16875–80 DOI: 10.1073/pnas.1012451107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Roddy GW, et al. , Action at a distance: systemically administered adult stem/progenitor cells (MSCs) reduce inflammatory damage to the cornea without engraftment and primarily by secretion of TNF-alpha stimulated gene/protein 6. Stem Cells, 2011. 29(10): p. 1572–9 DOI: 10.1002/stem.708. [DOI] [PubMed] [Google Scholar]
- 67.Oh JY, et al. , Intravenous mesenchymal stem cells prevented rejection of allogeneic corneal transplants by aborting the early inflammatory response. Mol Ther, 2012. 20(11): p. 2143–52 DOI: 10.1038/mt.2012.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu Y, et al. , Mesenchymal stem cells inhibit lipopolysaccharide-induced inflammatory responses of BV2 microglial cells through TSG-6. J Neuroinflammation, 2014. 11: p. 135 DOI: 10.1186/1742-2094-11-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang R, et al. , Anti-inflammatory and immunomodulatory mechanisms of mesenchymal stem cell transplantation in experimental traumatic brain injury. J Neuroinflammation, 2013. 10: p. 106 DOI: 10.1186/1742-2094-10-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bartosh TJ and Ylostalo JH, Preparation of anti-inflammatory mesenchymal stem/precursor cells (MSCs) through sphere formation using hanging-drop culture technique. Curr Protoc Stem Cell Biol, 2014. 28: p. Unit 2B.6. DOI: 10.1002/9780470151808.sc02b06s28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Akyurekli C, et al. , A systematic review of preclinical studies on the therapeutic potential of mesenchymal stromal cell-derived microvesicles. Stem Cell Rev Rep, 2015. 11(1): p. 150–60 DOI: 10.1007/s12015-014-9545-9. [DOI] [PubMed] [Google Scholar]
- 72.Kupcova Skalnikova H, Proteomic techniques for characterisation of mesenchymal stem cell secretome. Biochimie, 2013. 95(12): p. 2196–211 DOI: 10.1016/j.biochi.2013.07.015. [DOI] [PubMed] [Google Scholar]
- 73.Liang X, et al. , Paracrine mechanisms of mesenchymal stem cell-based therapy: current status and perspectives. Cell Transplant, 2014. 23(9): p. 1045–59 DOI: . [DOI] [PubMed] [Google Scholar]
- 74.Maumus M, Jorgensen C, and Noel D, Mesenchymal stem cells in regenerative medicine applied to rheumatic diseases: role of secretome and exosomes. Biochimie, 2013. 95(12): p. 2229–34 DOI: 10.1016/j.biochi.2013.04.017. [DOI] [PubMed] [Google Scholar]
- 75.Yeo RW, et al. , Mesenchymal stem cell: an efficient mass producer of exosomes for drug delivery. Adv Drug Deliv Rev, 2013. 65(3): p. 336–41 DOI: 10.1016/j.addr.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 76.Yu B, Zhang X, and Li X, Exosomes derived from mesenchymal stem cells. Int J Mol Sci, 2014. 15(3): p. 4142–57 DOI: 10.3390/ijms15034142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhang B, et al. , Mesenchymal stem cells secrete immunologically active exosomes. Stem Cells Dev, 2014. 23(11): p. 1233–44 DOI: 10.1089/scd.2013.0479. [DOI] [PubMed] [Google Scholar]
- 78.Conforti A, et al. , Microvescicles derived from mesenchymal stromal cells are not as effective as their cellular counterpart in the ability to modulate immune responses in vitro. Stem Cells Dev, 2014. 23(21): p. 2591–9 DOI: 10.1089/scd.2014.0091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kordelas L, et al. , MSC-derived exosomes: a novel tool to treat therapy-refractory graft-versus-host disease. Leukemia, 2014. 28(4): p. 970–3 DOI: 10.1038/leu.2014.41. [DOI] [PubMed] [Google Scholar]
- 80.Chen TS, et al. , Enabling a robust scalable manufacturing process for therapeutic exosomes through oncogenic immortalization of human ESC-derived MSCs. J Transl Med, 2011. 9: p. 47 DOI: 10.1186/1479-5876-9-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dunavin N, et al. , Mesenchymal Stromal Cells: What Is the Mechanism in Acute Graft-Versus-Host Disease? Biomedicines, 2017. 5(3) DOI: 10.3390/biomedicines5030039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lai RC, et al. , MSC secretes at least 3 EV types each with a unique permutation of membrane lipid, protein and RNA. J Extracell Vesicles, 2016. 5: p. 29828 DOI: 10.3402/jev.v5.29828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.E6(R2) Good Clinical Practice: Integrated Addendum to ICH E6(R1) Guidance for Industry, U.S.D.o.H.a.H. Services, Editor. 2018.
- 84.Przepiorka D, et al. , 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant, 1995. 15(6): p. 825–8. [PubMed] [Google Scholar]
- 85.MacMillan ML, DeFor TE, and Weisdorf DJ, What predicts high risk acute graft-versus-host disease (GVHD) at onset?: identification of those at highest risk by a novel acute GVHD risk score. Br J Haematol, 2012. 157(6): p. 732–41 DOI: 10.1111/j.1365-2141.2012.09114.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hartwell MJ, et al. , An early-biomarker algorithm predicts lethal graft-versus-host disease and survival. JCI Insight, 2017. 2(3): p. e89798 DOI: 10.1172/jci.insight.89798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rashidi A, et al. , Outcomes and Predictors of Response in Steroid-Refractory Acute Graft-versus-Host Disease. Biol Blood Marrow Transplant, 2019. 25(11): p. 2297–2302 DOI: 10.1016/j.bbmt.2019.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jagasia M, et al. , Risk factors for acute GVHD and survival after hematopoietic cell transplantation. Blood, 2012. 119(1): p. 296–307 DOI: 10.1182/blood-2011-06-364265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rashidi A, et al. , Steroids Versus Steroids Plus Additional Agent in Frontline Treatment of Acute Graft-versus-Host Disease: A Systematic Review and Meta-Analysis of Randomized Trials. Biol Blood Marrow Transplant, 2016. 22(6): p. 1133–1137 DOI: 10.1016/j.bbmt.2016.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zeiser R, et al. , Ruxolitinib for Glucocorticoid-Refractory Acute Graft-versus-Host Disease. N Engl J Med, 2020. DOI: 10.1056/NEJMoa1917635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Beyth S, et al. , Human mesenchymal stem cells alter antigen-presenting cell maturation and induce T-cell unresponsiveness. Blood, 2005. 105(5): p. 2214–9 DOI: 10.1182/blood-2004-07-2921. [DOI] [PubMed] [Google Scholar]
- 92.Herrero C and Perez-Simon JA, Immunomodulatory effect of mesenchymal stem cells. Braz J Med Biol Res, 2010. 43(5): p. 425–30 DOI: . [DOI] [PubMed] [Google Scholar]
- 93.Singer NG and Caplan AI, Mesenchymal stem cells: mechanisms of inflammation. Annu Rev Pathol, 2011. 6: p. 457–78 DOI: 10.1146/annurev-pathol-011110-130230. [DOI] [PubMed] [Google Scholar]
- 94.Aksu AE, et al. , Co-infusion of donor bone marrow with host mesenchymal stem cells treats GVHD and promotes vascularized skin allograft survival in rats. Clin Immunol, 2008. 127(3): p. 348–58 DOI: 10.1016/j.clim.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 95.Itakura S, et al. , Mesenchymal stem cells facilitate the induction of mixed hematopoietic chimerism and islet allograft tolerance without GVHD in the rat. Am J Transplant, 2007. 7(2): p. 336–46 DOI: 10.1111/j.1600-6143.2006.01643.x. [DOI] [PubMed] [Google Scholar]
- 96.Jeon MS, et al. , Xenoreactivity of human clonal mesenchymal stem cells in a major histocompatibility complex-matched allogeneic graft-versus-host disease mouse model. Cell Immunol, 2010. 261(1): p. 57–63 DOI: 10.1016/j.cellimm.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 97.Bartholomew A, et al. , Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol, 2002. 30(1): p. 42–8 DOI: 10.1016/s0301-472x(01)00769-x. [DOI] [PubMed] [Google Scholar]
- 98.Polchert D, et al. , IFN-gamma activation of mesenchymal stem cells for treatment and prevention of graft versus host disease. Eur J Immunol, 2008. 38(6): p. 1745–55 DOI: 10.1002/eji.200738129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Major-Monfried H, et al. , MAGIC biomarkers predict long-term outcomes for steroid-resistant acute GVHD. Blood, 2018. 131(25): p. 2846–2855 DOI: 10.1182/blood-2018-01-822957. [DOI] [PMC free article] [PubMed] [Google Scholar]


