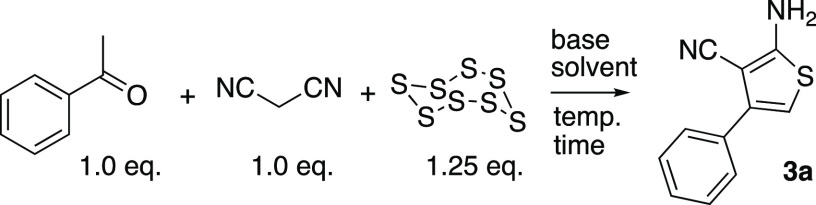Table 1. Optimization of the One-Pot Gewald Reaction.
| entry | base (equiv) | solvent | temp (°C) | time (h) | yield of 3a (%) |
|---|---|---|---|---|---|
| 1 | imidazole (0.1) | DMF | 60 | 18 | 10 |
| 2 | imidazole (0.1) | DMF | 80 | 24 | 14 |
| 3 | imidazole (0.25) | DMF | 60 | 24 | 18 |
| 4 | imidazole (0.25) | 2-Me-THF | 60 | 24 | 32 |
| 5 | imidazole (0.5) | 2-Me-THF | 60 | 24 | 36 |
| 6 | imidazole (1.0) | 2-Me-THF | 60 | 24 | 40 |
| 7 | imidazole (1.0) | 2-Me-THF | 80 | 24 | 40 |
| 8 | imidazole (1.0) | dioxane | 60 | 24 | 35 |
| 9 | morpholine (1.0) | 2-Me-THF | 60 | 24 | <1 |
| 10 | piperidine (1.0) | 2-Me-THF | 60 | 24 | <1 |
| 11 | morpholine (1.0) | DMF | 60 | 24 | <1 |
| 12 | piperidine (1.0) | DMF | 60 | 24 | <1 |
| 13 | proline (0.1) | DMF | 60 | 24 | 26 |
| 14 | proline (0.25) | DMF | 60 | 24 | 26 |
| 15 | imidazole (1.0) | 2-Me-THF | 60 | 24 | 57a |
Addition of sulfur 1.5 h after the reaction of acetophenone with malononitrile.