FIGURE 3.
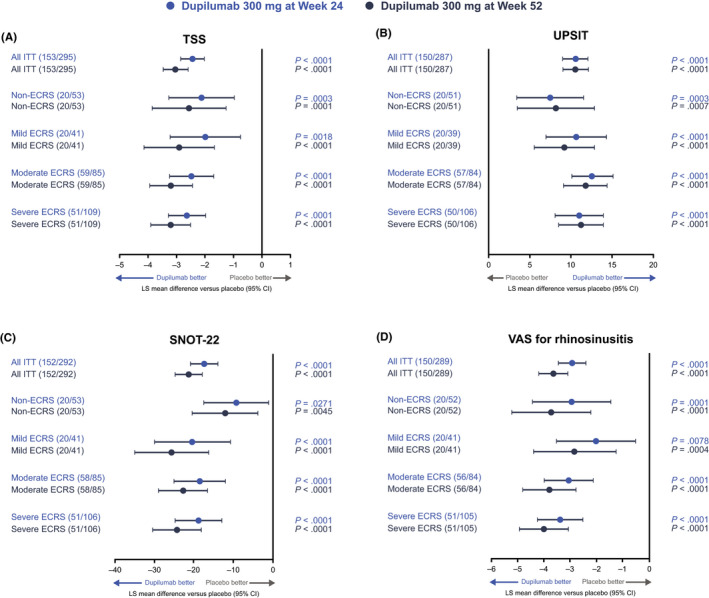
Effect of dupilumab 300 mg versus placebo on changes from baseline in secondary efficacy endpoints at weeks 24 and 52 by ECRS subgroup: (A) TSS, (B) UPSIT, (C) SNOT‐22, and (D) CRSwNP severity (VAS) scores. ECRS subgroups were defined according to the JESREC algorithm (Figure 1). Data for dupilumab 300 mg q2w and q2w–q4w treatment arms are pooled. Higher scores indicate greater disease severity, except for UPSIT, where higher scores indicate lower disease severity. CI, confidence interval; CRSwNP, chronic rhinosinusitis with nasal polyps; ECRS, eosinophilic chronic rhinosinusitis; ITT, intention‐to‐treat; JESREC, Japanese Epidemiological Survey of Refractory Eosinophilic Rhinosinusitis; LS, least squares; q2w, every 2 weeks; q4w, every 4 weeks; SNOT‐22, 22‐item Sinonasal Outcome Test; TSS, Total Symptom Score; UPSIT, University of Pennsylvania Smell Identification Test; VAS, visual analog scale
