Fig. 1. Lineage relationships of αβ T cell development in the thymus.
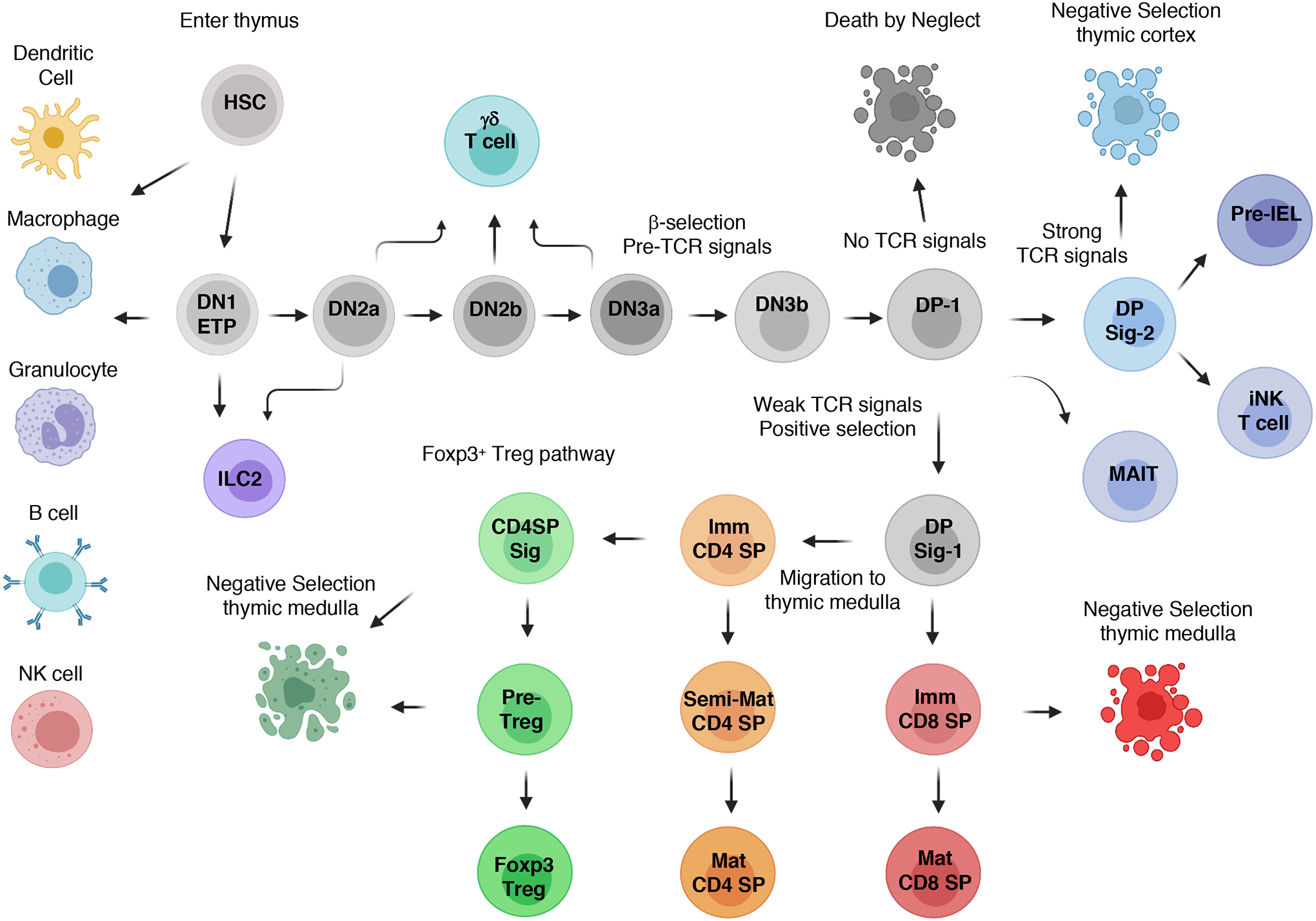
Fetal liver, followed by bone marrow-derived, hematopoietic stem cells (HSCs) enter the thymus, with most of the cells differentiating into early thymic progenitors (ETPs). Notch-1 signaling, in conjunction with proliferative and survival factors, induces progression to the double-negative 2 (DN2) stages and commitment to the T cell lineage, while limiting alternative cellular fates. TCF1 and other transcription factors induce the expression of genes encoding core TCR signaling components in DN2 thymocytes, whereas Rag1 and Rag2 expression enables the TRBV locus to be targeted for somatic recombination. Expression of a functional TCRβ chain allows DN3 thymocytes to progress through the β-selection checkpoint, which is followed by re-expression of RAG proteins to target the TRAV locus, leading to the generation of αβ TCR–expressing, resting double-positive 1 (DP-1) cells. The quality of TCR signaling derived from self-pMHC recognition at the DP-1 stage controls the initial stages of positive and negative selection, as well as the development of nonconventional T cells. CD4 and CD8 lineage choice occurs at the DP Sig-1 stage, which is followed by differentiation into immature CD4 or CD8 single-positive (SP) thymocytes. A second wave of negative selection can occur as immature CD4 and CD8 SP thymocytes migrate through the thymic medulla, with some immature CD4 SPs developing into Foxp3+ Treg cells. Note that only the major αβ thymocyte subsets are depicted.
