Fig. 5. Potential mechanisms of how basal self-reactivity contributes to T cell sensitivity to pathogens.
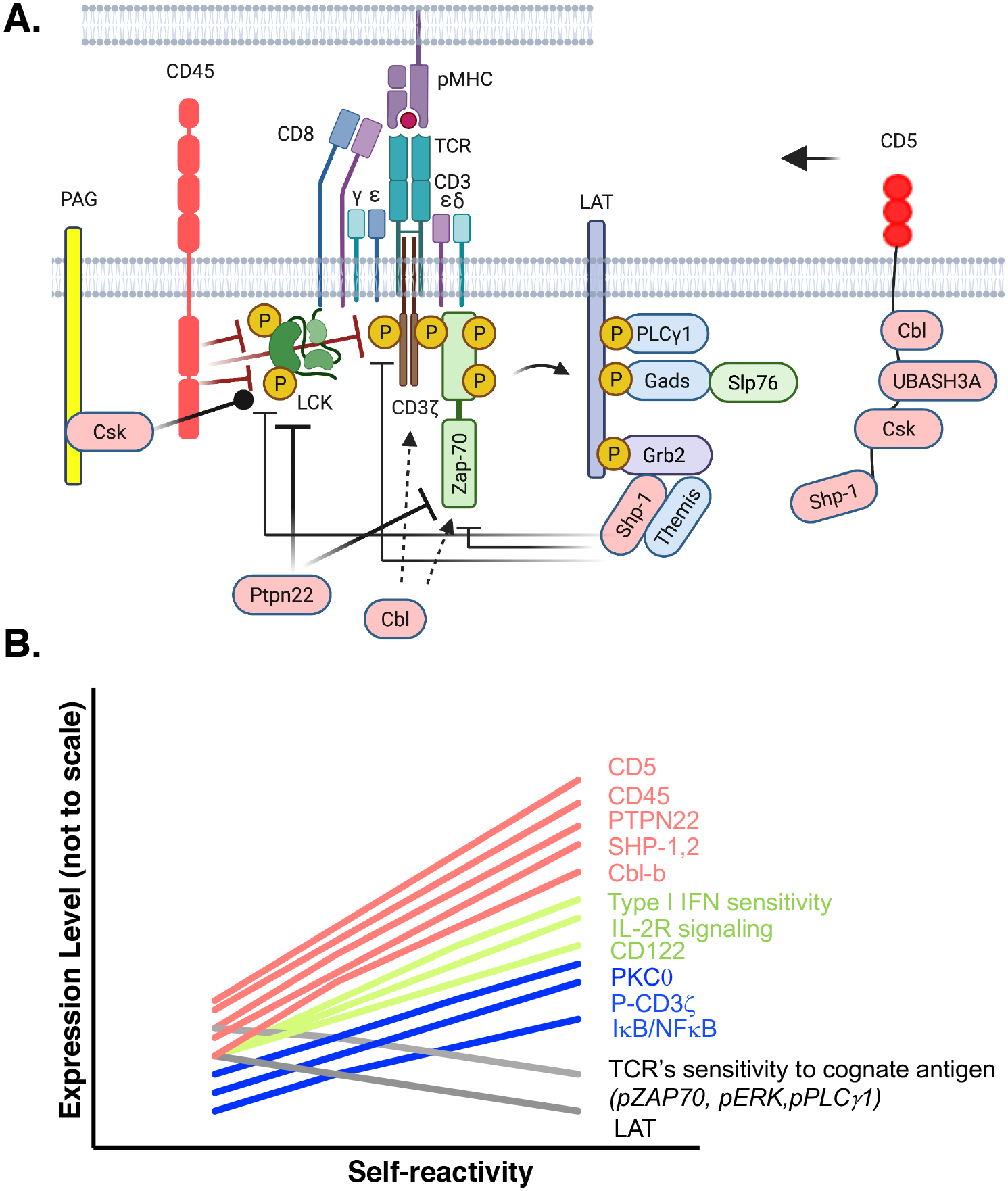
(A) High levels of self-reactivity increase the abundances of negative regulators of TCR signaling, including Shp-1, Shp-2, Cbl-b, CD5, Csk, Ptpn22, and CD45. CD45 dephosphorylates Lck and the CD3ζ chain to inhibit downstream signaling in the absence of antigen stimulation. The Lck kinase domain contains an activating tyrosine, Tyr394 and an inhibitory tyrosine, Tyr505. When Tyr394 is solely phosphorylated, Lck is fully active, whereas phosphorylation of Tyr505 inhibits Lck activity. By dephosphorylating both residues of Lck and Cd3ζ, CD45 limits, but does not abolish, Lck kinase function and counterbalances CD3ζ-chain phosphorylation. Csk is recruited to PAG and further limits Lck kinase activity by phosphorylating Lck Tyr505. Ptpn22 is a cytosolic phosphatase that once recruited to the plasma membrane can also inhibit Lck and Zap-70 activities. Shp-1 is recruited to the plasma membrane by different receptors, including CD5, and it inhibits TCR signaling by dephosphorylating Lck, Zap-70, and CD3ζ. Themis associates with LAT through Grb-2 and can inhibit SHP-1. c-Cbl and Cbl-b are E3 ubiquitin ligases that target ZAP-70 and CD3ζ for degradation and interrupt ZAP-70–CD3ζ interactions, respectively. CD5 is a membrane receptor that, when phosphorylated at cytosolic tyrosines by Lck, can recruit the inhibitory proteins c-Cbl, Cbl-b, UBASH3A, and Csk and bring them to the TCR complex. (B) Basal levels of self-reactivity influence TCR sensitivity through expression of negative regulators of TCR signals (Shp-1, Shp-2, Cbl, CD5, and CD45), which limit TCR signaling; phosphorylation of Zap-70, ERK, PLCγ1, and expression of LAT and IκB/NF-κB. Alterations further bias the T cell response to be receptive to cytokines, including IL-2 and type-1 IFN. The graph is for visualization purposes and relative differences in expression are not to scale.
